(MENAFN- PR Newswire)
NEW YORK, Sept. 19, 2024 /PRNewswire/ -- Report with market evolution powered by AI- The global hepatitis b and c diagnostics market size is estimated to grow by USD 3.02 billion from 2024-2028, according to Technavio. The market is estimated to grow at a CAGR of 8.09% during the forecast period. Growing demand for molecular diagnostics in diagnosis of HBV and HCV is driving market growth, with a trend towards advent of immunosensors in HBV and HCV diagnostic tests. However, low penetration of HBV and HCV diagnostic tests poses a challenge. Key market players include Abbott Laboratories, Bio Rad Laboratories Inc., bioMerieux SA, DAAN Gene Co. Ltd., DiaSorin SpA, Enzo Biochem Inc., F. Hoffmann La Roche Ltd., Grifols SA, Hologic Inc., MedMira Inc., OraSure Technologies Inc., Perkin Elmer Inc., QIAGEN NV, Quidelortho Corp., Randox Laboratories Ltd., Siemens AG, Sysmex Corp., and Xiamen Innovax Biotech Co. Ltd..
Continue Reading
Technavio has announced its latest market research report titled Global hepatitis B and C diagnostics market 2024-2028
Key insights into market evolution with AI-powered analysis. Explore trends, segmentation, and growth drivers- View the snapshot of this report
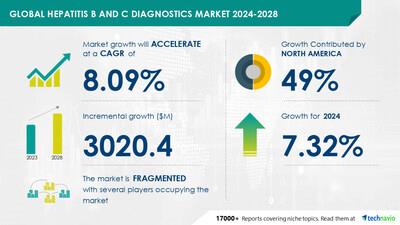
| Hepatitis B And C Diagnostics Market Scope |
| Report Coverage |
Details |
| Base year |
2023 |
| Historic period |
2018 - 2022 |
| Forecast period |
2024-2028 |
| Growth momentum & CAGR |
Accelerate at a CAGR of 8.09% |
| Market growth 2024-2028 |
USD 3020.4 million |
| Market structure |
Fragmented |
| YoY growth 2022-2023 (%) |
7.32 |
| Regional analysis |
North America, Europe, Asia, and Rest of World (ROW) |
| Performing market contribution |
North America at 49% |
| Key countries |
US, Germany, Japan, UK, and China |
| Key companies profiled |
Abbott Laboratories, Bio Rad Laboratories Inc., bioMerieux SA, DAAN Gene Co. Ltd., DiaSorin SpA, Enzo Biochem Inc., F. Hoffmann La Roche Ltd., Grifols SA, Hologic Inc., MedMira Inc., OraSure Technologies Inc., Perkin Elmer Inc., QIAGEN NV, Quidelortho Corp., Randox Laboratories Ltd., Siemens AG, Sysmex Corp., and Xiamen Innovax Biotech Co. Ltd. |
Market Driver
The Hepatitis B and C diagnostics market is experiencing growth due to the emergence of immunosensors for the detection of HBV and HCV. Immunosensors are devices that convert specific antibody-antigen interactions into measurable signals, enabling accurate and quick diagnosis. Biosensor technology, including electrochemical, piezoelectric, acoustic, magnetic, and optical sensors, is extensively used for HCV RNA and anti-HCV detection. Nanotechnology is also a significant trend in the market, with materials like carbon nanotubes, carbon nanofibers, and carbon nanorods being used as support materials for electrochemical immunosensors. These materials offer advantages such as large surface area, electrical conductivity, chemical stability, and biocompatibility. For instance, the University of Tehran developed nanotechnology-based immunosensors for HBV detection in 2014. The increasing need for better and quicker diagnosis and ongoing research activities are expected to drive the growth of the global hepatitis B and C diagnostics market.
The Hepatitis B and C diagnostics market is witnessing significant growth due to the increasing number of diagnostic tests for these liver diseases. Blood samples are the primary source for diagnosing these conditions through various methods like molecular diagnostics, nucleic acid assays, rapid diagnostic tests, and traditional methods using antibodies, antigens, enzymes, and proteins. Hospitals and diagnostic laboratories are the major consumers of these tests, with blood donation centers and clinical laboratories also contributing. Key technologies include Enzyme-linked immunosorbent assay (ELISA), Polymerase chain reaction (PCR), and Imaging tests like liver biopsy. Hepatitis B and C are public health concerns, with chronic infections affecting millions. Vaccination coverage and blood screening are essential for prevention and early detection. Reagents and kits, instruments, and skilled laboratory technicians are crucial for sample procurement, storage, transportation, and pathogen detection. Next-Generation Sequencing and Laboratory space are emerging trends. Cross-contamination is a concern, requiring stringent safety measures. HBV and HCV affect the liver, with HBV causing chronic infection in around 257 million people and HCV in 71 million. Diagnostics play a vital role in identifying these conditions for effective treatment.
Request Sample
of our comprehensive report now to stay ahead in the AI-driven market evolution!
Market Challenges
The global market for Hepatitis B and
C diagnostics faces challenges due to low diagnosis rates, particularly in low-income countries. Approximately 95% of individuals with these viruses remain undiagnosed, according to the World Hepatitis Alliance. This situation results from several factors, including insufficient awareness, limited diagnostic tools, and delivery issues. Government policies and funding are often absent, and the costly nature of these diagnostics further hinders their adoption. Moreover, complex treatment regimens negatively impact diagnosis rates. These issues may impede the growth of the Hepatitis B and C diagnostics market during the forecast period.
The Hepatitis B and
C diagnostics market faces several challenges in ensuring accurate and timely detection of these viruses. Blood tests, such as HBsAg testing for Hepatitis B and HCV RNA detection for Hepatitis C, are common diagnostic methods. However, challenges include the need for hospital and diagnostic laboratory resources, blood transfusion and donation safety, and nucleic acid testing complexities. Public health concerns around chronic HBV and HCV infections require ongoing awareness campaigns, particularly for adolescents and children. Imaging tests, liver biopsies, and clinical laboratories play crucial roles, but face challenges like sample procurement, storage, transportation, and cross-contamination. Next-Generation Sequencing and Pathogen detection technologies offer solutions, but require skilled laboratory technicians, sufficient laboratory space, and advanced equipment. Hepatitis B and C diagnostics are essential for effective treatment and management, making it a critical market to address these challenges. Immunoassays and PCR tests are widely used, but severe acute hepatitis cases may require additional diagnostic tools.
Discover how AI is revolutionizing market trends-
Get your access now!
Segment Overview
This hepatitis b and c diagnostics market report extensively covers market segmentation by
Type
1.1 Immunodiagnostics
1.2 NAT
Disease Type
2.1 Hepatitis B
2.2 Hepatitis C
Geography
3.1 North America
3.2 Europe
3.3 Asia
3.4 Rest of World (ROW)
1.1 Immunodiagnostics-
Immunodiagnostics, which include tests that detect antigens or antibodies through an enzyme-triggered color change, dominate the Hepatitis B and C diagnostics market. Approximately half of this market is represented by laboratory immunoassays, primarily used for detecting anti-hepatitis virus antibodies and viral surface and core antigens. The need for quantification diagnostic tests, which determine infection stages, is increasing. HCV and HBV viral core antigen testing is essential for accurate diagnosis and fuels market growth. Rapid diagnostic tests for HCV and advanced immunosensors are also driving market expansion. Immunodiagnostic tests, such as serological antibody assays, enzyme immunoassays (EIA), chemiluminescence immunoassays (CIA), and point-of-care (POC) rapid immunoassays, are used for Hepatitis B and C diagnosis. EIA is the most common initial HCV antibody test, while POC tests like the OraQuick HCV rapid antibody test offer quick diagnosis. Recombinant immunoblot assays (RIBA) help identify specific antibodies and distinguish between resolved infections and false positives. Immunoassay analyzers, which automate the testing process, and immunodiagnostic reagents, which contain all necessary materials for assay technologies, are essential instruments in the Hepatitis B and C diagnostics market. Automated immunoassay analyzers and integrated systems enable high-speed, multi-sample analysis. The market's growth is driven by the development of advanced immunodiagnostic technologies and the increasing demand for accurate and quick diagnoses.
Download a Sample
of our comprehensive report today to discover how AI-driven innovations are reshaping competitive dynamics
Research Analysis
The Hepatitis B and C diagnostics market encompasses a range of tests used to identify the presence of these viruses in individuals. These tests primarily involve analyzing a blood sample for the presence of specific antigens and antibodies, as well as nucleic acid assays for detecting viral RNA or DNA. Molecular diagnostics, including nucleic acid tests, have become increasingly important in the diagnosis of chronic HBV and HCV infections. Rapid diagnostic tests offer quick results, making them valuable in high-risk populations and resource-limited settings. Hepatitis B and C are significant public health concerns, with vaccination coverage varying globally. Chronic HBV and HCV infections can lead to liver damage and liver disease, making early diagnosis crucial. Diagnostic tests include HBsAg testing, HBV DNA testing, and HCV RNA detection, among others. Imaging tests and liver biopsy may also be used for further evaluation. Awareness campaigns are essential to encourage regular testing, particularly for those at higher risk, such as blood donors and recipients of blood transfusions.
Market Research Overview
Hepatitis B and C diagnostics involve various tests to detect the presence of these viruses in individuals. These tests include molecular diagnostics such as nucleic acid assays and rapid diagnostic tests that use antibodies, antigens, enzymes, or proteins to identify the viruses in a blood sample. Hepatitis B and C are major public health concerns, particularly in areas with low vaccination coverage and high rates of blood donations and transfusions. Diagnostic tests are crucial for identifying chronic HBV and HCV infections, which can lead to severe acute hepatitis and long-term liver damage. Diagnostic tests can be performed in hospitals, diagnostic laboratories, and clinical settings using instruments like ELISA, PCR, and imaging tests such as liver biopsy. Reagents and kits, as well as skilled laboratory technicians, are essential for sample procurement, storage, transportation, and pathogen detection. Next-Generation Sequencing is also used for advanced diagnosis. Nucleic acid tests are increasingly being used for blood screening to prevent cross-contamination. Awareness campaigns and education for adolescents and children are crucial for early detection and treatment. Immunoassays are commonly used for HBsAg testing, HBV DNA testing, and HCV RNA detection. Chronic HBV consultations and severe acute hepatitis cases require specialized diagnostic services.
Table of Contents:
1 Executive Summary
2 Market Landscape
3 Market Sizing
4 Historic Market Size
5 Five Forces Analysis
6 Market Segmentation
Type
Disease Type
Geography
North America
Europe
Asia
Rest Of World (ROW)
7 Customer Landscape
8 Geographic Landscape
9 Drivers, Challenges, and Trends
10 Company Landscape
11 Company Analysis
12 Appendix
About Technavio
Technavio is a leading global technology research and advisory company. Their research and analysis focuses on emerging market trends and provides actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions.
With over 500 specialized analysts, Technavio's report library consists of more than 17,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio's comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios.
Contacts
Technavio Research
Jesse Maida
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
Email:
[email protected]
Website:
SOURCE Technavio
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE?
440k+
Newsrooms &
Influencers
9k+
Digital Media
Outlets
270k+
Journalists
Opted In
GET STARTED
MENAFN19092024003732001241ID1108694436
Legal Disclaimer:
MENAFN provides the information “as is” without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the provider above.