(MENAFN- GlobeNewsWire - Nasdaq) The rising prevalence of urticaria is fueling market growth, with increased allergic responses and lifestyle shifts playing key roles. Advances in biologic treatments and a strong development pipeline from major companies address unmet patient needs. Expanding healthcare infrastructure and awareness efforts enhance treatment access, while research funding and digital innovations like telemedicine improve patient management and outcomes.New York, USA, Nov. 04, 2024 (GLOBE NEWSWIRE) -- Urticaria Clinical Trial Pipeline Accelerates: 20+ Leading Companies Pioneering New Treatments | DelveInsight
The rising prevalence of urticaria is fueling market growth, with increased allergic responses and lifestyle shifts playing key roles. Advances in biologic treatments and a strong development pipeline from major companies address unmet patient needs. Expanding healthcare infrastructure and awareness efforts enhance treatment access, while research funding and digital innovations like telemedicine improve patient management and outcomes.
DelveInsight's ' Urticaria Pipeline Insight 2024 ' report provides comprehensive global coverage of pipeline urticaria therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the urticaria pipeline domain.
Key Takeaways from the Urticaria Pipeline Report
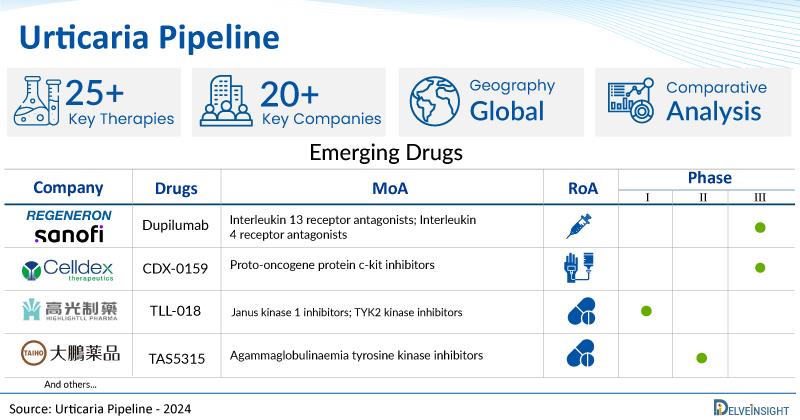
DelveInsight's urticaria pipeline report depicts a robust space with 20+ active players working to develop 25+ pipeline urticaria drugs. Key urticaria companies such as Regeneron Pharmaceuticals, Taiho Pharmaceutical Co., Ltd, Allakos Inc, Yuhan Corporation, United BioPharma, Hangzhou Highlightll Pharmaceutical Co., Ltd, Kiniksa Pharmaceuticals, Ltd., Celldex Therapeutics, Longbio Pharma, Celltrion, Enanta Pharmaceuticals, Evommune, and others are evaluating new urticaria drugs to improve the treatment landscape. Promising pipeline urticaria therapies such as Dupilumab, TAS5315, AK006, YH35324, UB-221, TLL-018, KPL-716, AK002, CDX-0159, LP-003, CT-P39, Research programme: Chronic urticaria therapeutics, EVO756, and others are under different phases of urticaria clinical trials. In September 2024, Regeneron Pharmaceuticals and Sanofi announced that a Dupixent (dupilumab) confirmatory Phase III trial (LIBERTY-CUPID Study C) met the primary and key secondary endpoints for the investigational treatment of patients with uncontrolled, biologic-naïve chronic spontaneous urticaria (CSU) receiving background therapy with antihistamines. In September 2024, Evommune announced the enrollment of the first patient in a Phase II trial of EVO756 in adults with CIndU. In June 2024, Celldex Therapeutics announced data demonstrating that barzolvolimab profoundly improves angioedema at 12 weeks in the Company's Phase II clinical trial in chronic spontaneous urticaria (CSU). In May 2024, Novartis announced new data that confirm the long-term efficacy and safety of remibrutinib, a highly selective Bruton's tyrosine kinase (BTK) inhibitor, in chronic spontaneous urticaria (CSU)1. In March 2024, Jasper Therapeutics announced that the first patient had been dosed in Jasper's Phase Ib/IIa (SPOTLIGHT) clinical study of subcutaneous briquilimab for the treatment of CIndU. In February 2024, ARS Pharmaceuticals announced positive efficacy results in its phase II inpatient chronic spontaneous urticaria study with neffy (epinephrine nasal spray), an investigational new drug.
Request a sample and discover the recent advances in urticaria drugs @ Urticaria Pipeline Report
The urticaria pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage urticaria drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the urticaria clinical trial landscape.
Urticaria Overview
Urticaria, also referred to as hives, is a skin condition marked by the sudden formation of raised, itchy welts on the skin, known as wheals. These welts can vary in size, from small spots to larger patches, and can appear anywhere on the body. Urticaria is a common condition, affecting up to 20% of people at some point in their lives. It can be either acute, lasting less than six weeks, or chronic, lasting more than six weeks. Acute urticaria is often caused by allergic reactions, while chronic urticaria can be more difficult to diagnose and manage due to its persistent nature.
The main urticaria symptom is the presence of welts, which are usually red, pink, or flesh-toned and are often surrounded by a red ring. They can change size and shape and may migrate, disappearing from one area and reappearing in another within minutes or hours. The welts are typically itchy and may cause a burning or stinging feeling. In some instances, urticaria may lead to angioedema, which causes swelling beneath the skin, especially around the eyes, lips, and genitals. Severe cases can result in difficulty breathing or swallowing, requiring urgent medical care.
Urticaria arises when skin cells known as mast cells release histamine and other chemicals into the bloodstream. This can be triggered by various factors, such as allergic reactions to food, medications, insect stings, or contact with specific plants or animals. Non-allergic triggers may include infections, stress, physical exertion, temperature changes, pressure on the skin, and sun exposure. In cases of chronic urticaria, the exact cause often remains unclear, though autoimmune mechanisms are suspected in many cases. Histamine release causes fluid leakage from blood vessels into nearby tissues, leading to the characteristic swelling and itching.
Diagnosis is primarily clinical, based on the appearance of skin lesions and the patient's history. For acute urticaria, identifying and removing the trigger often resolves the issue. In chronic cases, a thorough medical history and physical exam are important, with additional tests like blood work, allergy testing, or skin biopsies conducted to rule out other conditions and determine possible causes. Sometimes, controlled challenge tests may be performed to identify specific triggers.
The goal of urticaria treatment is to alleviate symptoms and, if possible, address the root cause. Antihistamines are the first-line treatment, effectively reducing itching and swelling. More severe cases may require short-term oral corticosteroids. Chronic urticaria that doesn't respond to antihistamines might be treated with immune-modulating drugs, such as omalizumab, a monoclonal antibody. Avoiding known triggers is essential in managing the condition, and in cases where an allergen is identified, desensitization therapy may be useful. Lifestyle changes, such as stress reduction and avoiding extreme temperatures, can also help manage symptoms.
Find out more about urticaria drugs @ Urticaria Analysis
A snapshot of the Pipeline Urticaria Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| Dupilumab | Regeneron Pharmaceuticals/Sanofi | III | Interleukin 13 receptor antagonists; Interleukin 4 receptor antagonists | Subcutaneous |
| CDX-0159 | Celldex Therapeutics Inc | III | Proto-oncogene protein c-kit inhibitors | Intravenous |
| TLL-018 | TLL Pharmaceutical | I | Janus kinase 1 inhibitors; TYK2 kinase inhibitors | Oral |
| TAS5315 | Taiho Pharmaceutical | II | Agammaglobulinaemia tyrosine kinase inhibitors | Oral |
| UB-221 | United BioPharma | II | IgE receptor antagonists; Immunomodulators | Intravenous |
| AK006 | Allakos Inc. | I | Mast cell inhibitors; Phagocyte stimulants | Intravenous |
| YH35324 | Yuhan | I | Immunoglobulin E inhibitors | Subcutaneous |
Learn more about the emerging urticaria therapies @ Urticaria Clinical Trials
Urticaria Therapeutics Assessment
The urticaria pipeline report proffers an integral view of the emerging urticaria therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Urticaria Pipeline Report
Coverage : Global Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III Therapeutics Assessment By Route of Administration: Oral, Intravenous, Subcutaneous, Parenteral, Topical Therapeutics Assessment By Molecule Type : Recombinant fusion proteins, Small molecule, Monoclonal antibody, Peptide, Polymer, Gene therapy Therapeutics Assessment By Mechanism of Action : Interleukin 13 receptor antagonists, Interleukin 4 receptor antagonists, Proto-oncogene protein c-kit inhibitors, Agammaglobulinaemia tyrosine kinase inhibitors, IgE receptor antagonists, Immunomodulators, Mast cell inhibitors; Phagocyte stimulants, Immunoglobulin E inhibitors, Janus kinase 1 inhibitors, TYK2 kinase inhibitors Key Urticaria Companies : Regeneron Pharmaceuticals, Taiho Pharmaceutical Co., Ltd, Allakos Inc, Yuhan Corporation, United BioPharma, Hangzhou Highlightll Pharmaceutical Co., Ltd, Kiniksa Pharmaceuticals, Ltd., Celldex Therapeutics, Longbio Pharma, Celltrion, Enanta Pharmaceuticals, Evommune, and others Key Urticaria Pipeline Therapies : Dupilumab, TAS5315, AK006, YH35324, UB-221, TLL-018, KPL-716, AK002, CDX-0159, LP-003, CT-P39, Research programme: Chronic urticaria therapeutics, EVO756, and others
Dive deep into rich insights for new urticaria treatments, visit @ Urticaria Drugs
Table of Contents
| 1. | Urticaria Pipeline Report Introduction |
| 2. | Urticaria Pipeline Report Executive Summary |
| 3. | Urticaria Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Urticaria Clinical Trial Therapeutics |
| 6. | Urticaria Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Urticaria Pipeline: Late-Stage Products (Phase III) |
| 8. | Urticaria Pipeline: Mid-Stage Products (Phase II) |
| 9. | Urticaria Pipeline: Early-Stage Products (Phase I) |
| 10. | Urticaria Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Urticaria Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Urticaria Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the urticaria pipeline therapeutics, reach out @ Urticaria Therapeutics
Related Reports
Urticaria Epidemiology Forecast
Urticaria Epidemiology Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, and urticaria epidemiology trends.
Chronic Spontaneous Urticaria Market
Chronic Spontaneous Urticaria Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key CSU companies, including Roche, Novartis, Sanofi, Regeneron, AstraZeneca, Amgen, Taiho Pharmaceutical, among others.
Chronic Spontaneous Urticaria Pipeline
Chronic Spontaneous Urticaria Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, including clinical and non-clinical stage products, and the key CSU companies, including United BioPharma, Teva Pharmaceuticals Development, Inc., Amgen, Novartis Pharmaceuticals, Allakos Inc., Sanofi, Celltrion, Celldex Therapeutics, MICROBIO GROUP., Escient Pharmaceuticals, Jasper Therapeutics, Glenmark Pharmaceuticals, Taiho Pharmaceuticals, ValenzaBio, Carna Biosciences, Servier , among others.
Chronic Inducible Urticaria Market
Chronic Inducible Urticaria Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key CIU companies, including Novartis Pharmaceuticals, Jasper Therapeutics, Inc., Celldex Therapeutics, among others.
Chronic Inducible Urticaria Pipeline
Chronic Inducible Urticaria Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, including clinical and non-clinical stage products, and the key CIU companies, including Celldex Therapeutics, Jasper Therapeutics , among others.
DelveInsight's Pharma Competitive Intelligence Service : Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Consulting Services
Healthcare Conference Coverage
Pipeline Assessment
Healthcare Licensing Services
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn
CONTACT: Contact Us
Shruti Thakur
...
+14699457679

MENAFN04112024004107003653ID1108849221