(MENAFN- GlobeNewsWire - Nasdaq)
New non-human primate data show highly selective in vivo delivery of mRNA to T cells with a cell-targeted lipid nanoparticle (ctLNP)
CAMBRIDGE, Mass., Oct. 22, 2024 (GLOBE NEWSWIRE) -- Generation Bio Co. (Nasdaq:GBIO) a biotechnology company innovating genetic medicines for people living with rare and prevalent diseases, today presented data on its cell-targeted lipid nanoparticle (ctLNP) and immune-quiet DNA (iqDNA) platforms at the European Society of Gene and Cell Therapy (ESGCT) 31st Annual Congress.
The company presented data showing that a single IV dose of its ctLNP delivered mRNA encoding a reporter protein to a majority of circulating T cells in non-human primates (NHPs). In the study, conducted as part of Generation Bio's partnership with Moderna, Inc., NHPs received a single 1 mg/kg dose of ctLNP-mRNA, which transduced most T cells with balanced distribution to CD4+ and CD8+ cells. Untargeted LNPs did not elicit T cell transduction. The biodistribution of ctLNP-mRNA to the liver, spleen, lung, and monocytes was minimal. These results in NHPs confirm and improve on results from prior murine studies.
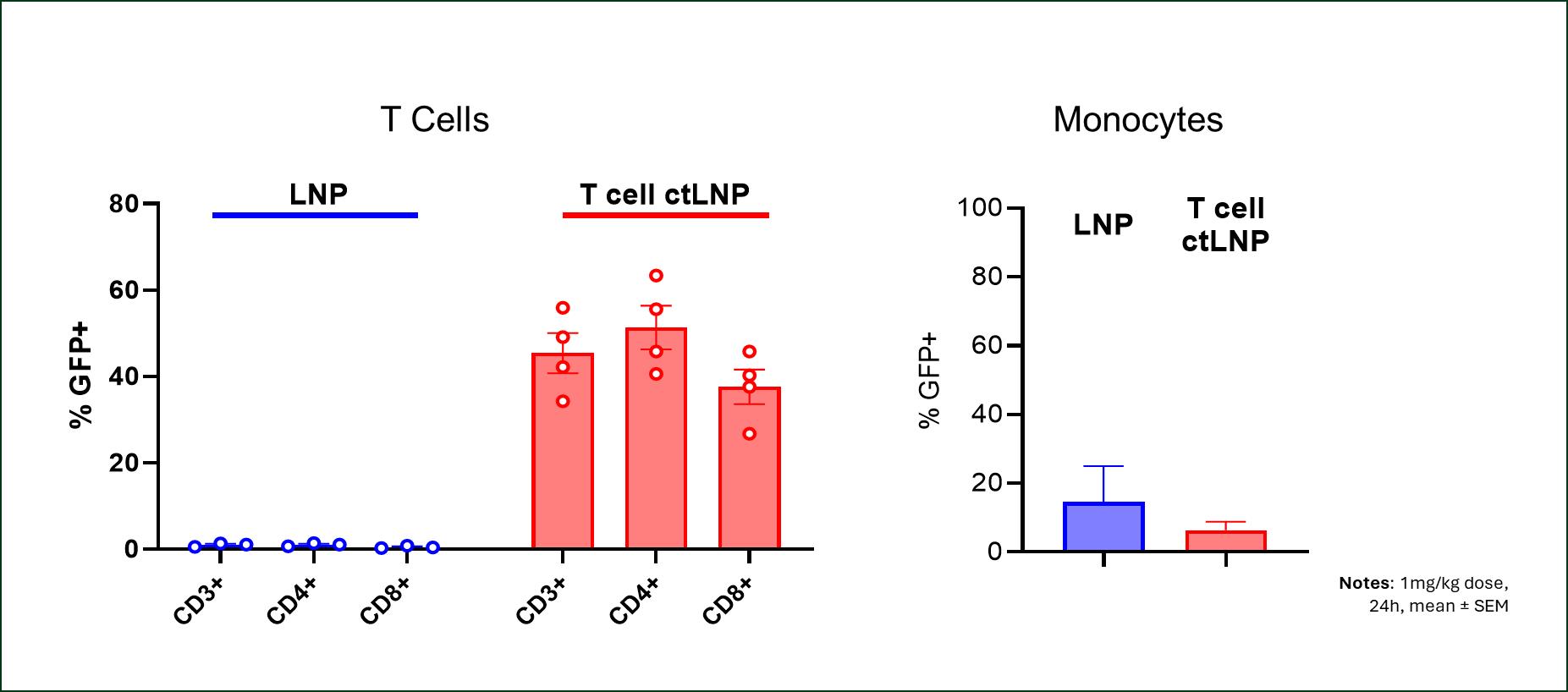
Figure 1: Highly specific transduction of CD3+, CD4+, and CD8+ cells with T cell targeted ctLNP achieved following 1 mg/kg dose of ctLNP-mRNA, with no monocyte uptake above baseline
“The robust and selective in vivo delivery to T cells in an NHP model gives us the conviction to pursue development of T cell targeted in vivo genetic medicines,” said Matt Stanton, Ph.D., chief science officer of Generation Bio.
The company also presented updates on iqDNA, which is a partially single-stranded DNA molecule that avoids recognition by primary DNA sensors in mice and NHPs. Recent work on the iqDNA platform has focused on methods of improving protein expression, including priming second strand synthesis by encoding the promoter region as double-stranded and stabilizing iqDNA against degradation by exonucleases.
Dr. Stanton added:“Our research on iqDNA continues to yield important insights into the characterization and behavior of this new molecule. We are pursuing increasingly focused areas of research to make iqDNA ready for the development of novel genetic medicines.”
To learn more about the data presented at ESGCT, visit the Scientific Presentations page of Generation Bio's website.
Forward-Looking Statements
Any statements in this press release about future expectations, plans and prospects for the company, including statements about the company's strategic plans or objectives, technology platforms, research and clinical development plans, and preclinical data and other statements containing the words“believes,”“anticipates,”“plans,”“expects,” and similar expressions, constitute forward-looking statements within the meaning of The Private Securities Litigation Reform Act of 1995. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including: uncertainties inherent in the identification and development of product candidates, including the conduct of research activities, the initiation and completion of preclinical studies and clinical trials and clinical development of the company's product candidates; uncertainties as to the availability and timing of results from preclinical studies and clinical trials; uncertainties regarding our novel platforms and related technologies; whether results from preclinical studies will be predictive of the results of later preclinical studies and clinical trials; challenges in the manufacture of genetic medicine products; whether the company's cash resources are sufficient to fund the company's operating expenses and capital expenditure requirements for the period anticipated; as well as the other risks and uncertainties set forth in the“Risk Factors” section of the company's most recent annual report on Form 10-Kand quarterly report on Form 10-Q, which are on file with the Securities and Exchange Commission, and in subsequent filings the company may make with the Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent the company's views as of the date hereof. The company anticipates that subsequent events and developments will cause the company's views to change. However, while the company may elect to update these forward-looking statements at some point in the future, the company specifically disclaims any obligation to do so. These forward-looking statements should not be relied upon as representing the company's views as of any date subsequent to the date on which they were made.
About Generation Bio
Generation Bio is innovating non-viral genetic medicines to provide durable and redosable treatments for hundreds of millions of patients living with rare and prevalent diseases. The company is developing two distinct and complementary platforms: a potent, highly selective cell-targeted lipid nanoparticle (ctLNP) delivery system and a novel immune-quiet DNA (iqDNA) cargo produced by a scalable capsid-free manufacturing process that uses proprietary cell-free rapid enzymatic synthesis (RES). With these platforms, Generation Bio aims to develop the next wave of non-viral genetic medicines to support its mission to extend the reach of genetic medicine to more people living with more diseases, around the world.
For more information, please visit .
Investors and Media Contact
Maren Killackey
Generation Bio
...
857-371-4638
A photo accompanying this announcement is available at

MENAFN22102024004107003653ID1108805483
Legal Disclaimer:
MENAFN provides the information “as is” without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the provider above.