
403
Sorry!!
Error! We're sorry, but the page you were looking for doesn't exist.
Creative Diagnostics Launches Reliable Hemagglutination Assay For Virus And Antibody Detection
(MENAFN- ForPressRelease)
As an expert in providing solutions to assist virology and microbiology research, Creative Diagnostics is proud to announce the launch of its Hemagglutination Assay (HA) services for effective virus and antibody detection. The new anti-virus testing solution offers a valuable tool for researchers working on influenza virus characterization, vaccine development, and general viral diagnostics.
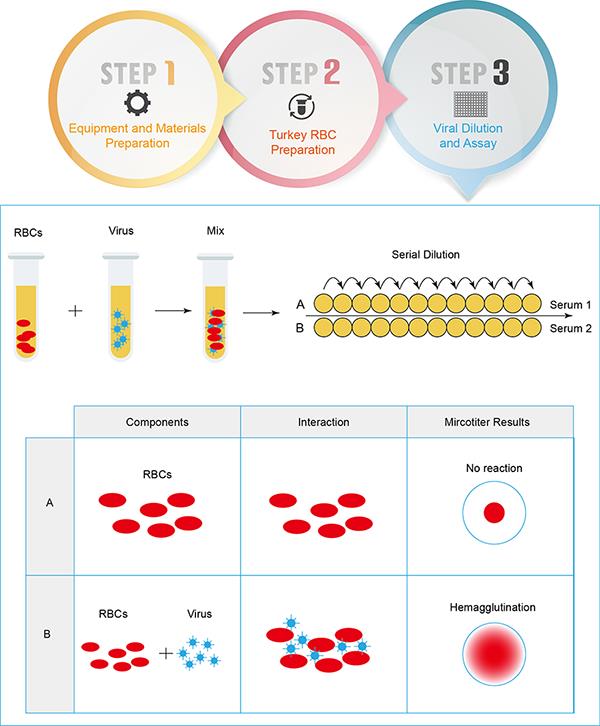
Certain viruses agglutinate red blood cells in certain animals, a process known as viral hemagglutination. HA test is designed to infer the presence of the virus in the material being tested and to determine whether this response is specific. It is a well-established method that is widely used in influenza vaccine, diagnostic and surveillance to measure virus and antibody titers and to monitor influenza subtypes.
Hemagglutination occurs when the hemagglutinin protein on the surface of the influenza virus binds to a serine on red blood cells (RBCs). Similarly, when sufficient numbers of virus and RBCs are present, the virus particles act as a bridging agent, forming a network that connects the RBCs, resulting in hemocoagulation.
The hemagglutination assay is the most commonly used method for detecting influenza virus, adenovirus, rabies virus, and many other microorganisms. It is the basis for a wide range of veterinary and life science diagnostic tests. HA analysis is simple, uses relatively inexpensive and commonly available instruments and consumables, and provides results within a few hours. The method has been well validated in many laboratories around the world and has a degree of reliability, comparability and standardization.
In addition, the ability of viruses to agglutinate RBCs can be inhibited by specific antibodies, leading to the development of the hemagglutination inhibition (HAI) assay. The HA-HAI test allows the identification of unknown viruses in known sera and the titration of antibodies in unknown sera with known viruses by determining the level of the corresponding antibodies. The hemagglutination assay, used in conjunction with interactions, produces a visible network showing the relative concentrations of viruses and antiviral antibodies.
Creative Diagnostics now offers Hemagglutination Assay services and customized early detection solutions to life science researchers focused on antiviral and infectious disease diagnostics. The research team combines infectious disease and analytical expertise to provide customers with the most powerful portfolio of antiviral and antimicrobial in vitro testing services.
For example, the Creative Diagnostics team uses a wide range of erythrocytes from different species (chicken, guinea pig, goose, horse, cow, sheep, rabbit, and human A, B, AB, and O) and standardizes experiments using reference standards. In addition, because the aggregation capacity of RBCs declines over time, the team prepares fresh RBCs each time to ensure reproducible results.
Creative Diagnostics offers a comprehensive portfolio of antiviral testing services, including the Hemagglutination Assay. For more information on how this assay can benefit your research, please visit
About Creative Diagnostics
Headquartered in New York, Creative Diagnostics is a consulting and experimental service provider specializing in virology and microbiology. The company provides comprehensive solutions to conquer obstacles in virology and microbiology research, from high-security infrastructure provision, biosafety regulation elucidation, to expert viral system assistance.
Certain viruses agglutinate red blood cells in certain animals, a process known as viral hemagglutination. HA test is designed to infer the presence of the virus in the material being tested and to determine whether this response is specific. It is a well-established method that is widely used in influenza vaccine, diagnostic and surveillance to measure virus and antibody titers and to monitor influenza subtypes.
Hemagglutination occurs when the hemagglutinin protein on the surface of the influenza virus binds to a serine on red blood cells (RBCs). Similarly, when sufficient numbers of virus and RBCs are present, the virus particles act as a bridging agent, forming a network that connects the RBCs, resulting in hemocoagulation.
The hemagglutination assay is the most commonly used method for detecting influenza virus, adenovirus, rabies virus, and many other microorganisms. It is the basis for a wide range of veterinary and life science diagnostic tests. HA analysis is simple, uses relatively inexpensive and commonly available instruments and consumables, and provides results within a few hours. The method has been well validated in many laboratories around the world and has a degree of reliability, comparability and standardization.
In addition, the ability of viruses to agglutinate RBCs can be inhibited by specific antibodies, leading to the development of the hemagglutination inhibition (HAI) assay. The HA-HAI test allows the identification of unknown viruses in known sera and the titration of antibodies in unknown sera with known viruses by determining the level of the corresponding antibodies. The hemagglutination assay, used in conjunction with interactions, produces a visible network showing the relative concentrations of viruses and antiviral antibodies.
Creative Diagnostics now offers Hemagglutination Assay services and customized early detection solutions to life science researchers focused on antiviral and infectious disease diagnostics. The research team combines infectious disease and analytical expertise to provide customers with the most powerful portfolio of antiviral and antimicrobial in vitro testing services.
For example, the Creative Diagnostics team uses a wide range of erythrocytes from different species (chicken, guinea pig, goose, horse, cow, sheep, rabbit, and human A, B, AB, and O) and standardizes experiments using reference standards. In addition, because the aggregation capacity of RBCs declines over time, the team prepares fresh RBCs each time to ensure reproducible results.
Creative Diagnostics offers a comprehensive portfolio of antiviral testing services, including the Hemagglutination Assay. For more information on how this assay can benefit your research, please visit
About Creative Diagnostics
Headquartered in New York, Creative Diagnostics is a consulting and experimental service provider specializing in virology and microbiology. The company provides comprehensive solutions to conquer obstacles in virology and microbiology research, from high-security infrastructure provision, biosafety regulation elucidation, to expert viral system assistance.
Company :-Creative Diagnostics
User :- Thomas Schmitt
Email :...
Url :-
Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.
















Comments
No comment