NASA NOW EQUIPPED WITH ECHOLIGHT DEVICES FOR BONE HEALTH MONITORING IN SPACEFLIGHT SIMULATIONS
Date
10/21/2024 7:17:00 AM
(MENAFN- PR Newswire)
HOUSTON, Oct. 21, 2024 /PRNewswire/ --
Echolight, a global innovator in bone densitometry, announced that NASA is using its proprietary REMS (Radiofrequency Echographic Multi Spectrometry) technology to
monitor bone changes in a human bedrest study
and to assess the effects of unloading on bone mass given the paramount importance of Bone health in space too.
Continue Reading
NASA is using REMS Technology from Echolight to monitor bone changes in a human bedrest study
Post this
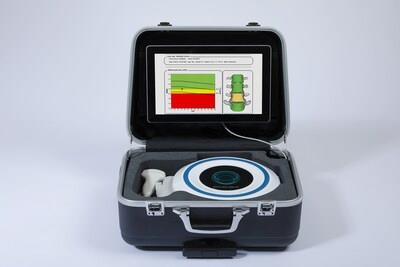
Echolight ECHOS portable solution

Echolight Medical
Through a simple ultrasound scan of axial anatomical sites such as the spine and femur, the REMS innovative technology measures bone density and microarchitecture without using the radiation of traditional x-ray scans. This radiation-free technology allows scientists to perform repeated scans for monitoring bone health over time. In addition, the ability to automatically exclude artifacts commonly present with other densitometry procedures yields a highly reliable diagnostic assessment.
"Echolight is pleased with this additional milestone in the US adoption of REMS devices, where the diagnostic system for the unique, personalized and patient-centered assessment of bone health has registered a very high interest among the US medical community sharing the intent to reduce fragility fractures," explained Sergio Casciaro, Echolight S.p.A. CEO and founder.
Prof. Aenor Sawyer, director of UCSF Skeletal Health Service and of UC Space Health, reported "Echolight technology provides a number of potential benefits for possible use during spaceflight missions including its portability (the unit is the size of a small suitcase), fast scan time of under 10 minutes, edge analytics, and the ability to scan frequently with no harmful radiation effects. NASA is currently unable to assess the bone loss of each astronaut occurring in spaceflight and make real-intime adjustments to their countermeasures. This will be increasingly important on longer missions such as those to the Moon and Mars, and REMS could potentially provide this critical in-mission information."
Echolight
was established in Italy with head office in Lecce, and in 2020 opened its offices in the United States. Since then, its sales network has grown to more than 40 distributors and several hundreds of satisfied customers across the world who have adopted the innovative R.E.M.S. technology for assessing the bone condition of their patients.
Created as a spin-off of the Italian National Research Council, Echolight is at the forefront of medical innovation, introducing new cutting-edge solutions to contribute to human wellbeing. It has been awarded all the standard certificates: UNI CEI EN ISO 13485: 2016, Quality Management System UNI EN ISO 9001: 2015, CE mark, FDA clearance, and other major certificates worldwide for innovative solutions considered as the best practice in bone health for personalized medicine.
SOURCE Echolight Medical
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE?
440k+
Newsrooms &
Influencers
9k+
Digital Media
Outlets
270k+
Journalists
Opted In
GET STARTED
MENAFN21102024003732001241ID1108802453

Legal Disclaimer:
MENAFN provides the information “as is” without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the provider above.