
KRAS Inhibitors Market: New Treatments Are Set To Change Cancer Care | Delveinsight
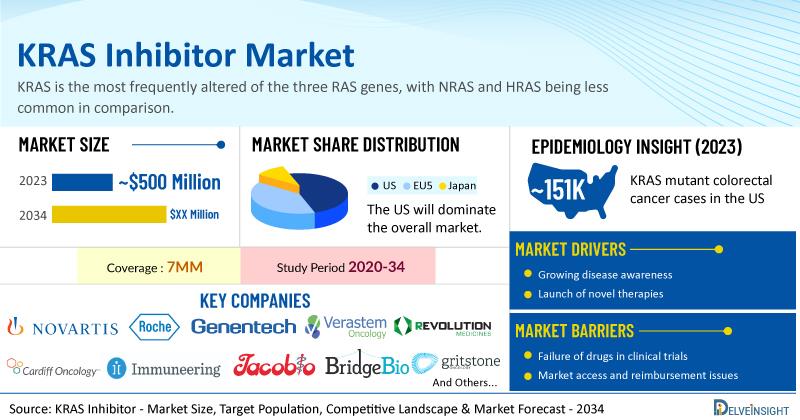
| KRAS Inhibitor Report Metrics | Details |
| Study Period | 2020–2034 |
| KRAS Inhibitor Report Coverage | 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| KRAS Inhibitors Market Size in 2023 | USD 500 Million |
| Key KRAS Inhibitor Companies | Novartis, Roche, Genentech, Verastem Oncology, Revolution Medicines, Cardiff Oncology, Immuneering Corporation, Jacobio Pharmaceuticals, BridgeBio Pharma (Navire Pharma), Mirati Therapeutics, Deciphera Pharmaceuticals, Elicio Therapeutics, InventisBio, Gritstone bio, D3 Bio, Amgen, and others |
| Key KRAS Inhibitors | JDQ443, Divarasib, Avutometinib (VS-6766), RMC-4630, Onvansertib, IMM-1-104, Glecirasib (JAB-21822), BBP-398, MRTX1133, DCC-3116, ELI-002, D-1553, SLATE-KRAS, D3S-001, LUMAKRAS/LUMYKRAS (sotorasib), KRAZATI (adagrasib), and others |
Scope of the KRAS Inhibitor Market Report
- KRAS Inhibitor Therapeutic Assessment: KRAS Inhibitor current marketed and emerging therapies KRAS Inhibitor Market Dynamics: Conjoint Analysis of Emerging KRAS Inhibitor Drugs Competitive Intelligence Analysis: SWOT analysis and Market entry strategies Unmet Needs, KOL's views, Analyst's views, KRAS Inhibitor Market Access and Reimbursement
Discover more about KRAS inhibitor drugs in development @ KRAS Inhibitor Clinical Trials
Table of Contents
| 1 | Key Insights |
| 2 | Report Introduction |
| 3 | Key Highlights of the Report |
| 4 | Executive Summary of KRAS Inhibitors |
| 5 | Key Events |
| 6 | Epidemiology and Market Forecast Methodology |
| 7 | KRAS-inhibitors Market Overview at a Glance |
| 7.1 | Market Share (%) Distribution of KRAS-inhibitors by Therapies in 2023 |
| 7.2 | Market Share (%) Distribution of KRAS-inhibitors by Therapies in 2034 |
| 7.3 | Market Share (%) Distribution of KRAS-inhibitors by Indications in 2023 |
| 7.4 | Market Share (%) Distribution of KRAS-inhibitors by Indications in 2034 |
| 8 | Disease Background and Overview |
| 8.1 | Introduction |
| 8.2 | Clinical Significance |
| 8.2.1 | RAS Oncogene and Carcinogenesis as a Multistep Process |
| 8.2.2 | KRAS Mutation as a Prognostic Biomarker |
| 8.2.3 | KRAS Mutation and Personalized Medicine |
| 8.3 | Diagnosis |
| 8.4 | Biomarker testing for KRAS mutation |
| 8.4.1 | NSCLC |
| 8.4.2 | Colorectal Cancer |
| 8.4.3 | Pancreatic Cancer |
| 8.5 | Clinical Relevance of KRAS Mutation by Cancer Type |
| 8.5.1 | Pancreatic Cancer |
| 8.5.2 | Colorectal Cancer |
| 8.5.2.1 | Tumor-based Tests for KRAS Gene Mutations |
| 8.5.3 | Lung Cancer |
| 8.5.4 | Ovarian Cancer |
| 8.5.5 | Hepatocellular Carcinoma (HCC) |
| 9 | Treatment |
| 9.1 | Treatment of NSCLC |
| 9.1.1 | Surgery |
| 9.1.2 | Radiofrequency Ablation (RFA) |
| 9.1.3 | Radiation Therapy |
| 9.1.4 | Chemotherapy |
| 9.1.5 | Immunotherapy |
| 9.2 | Treatment of Pancreatic Cancer |
| 9.2.1 | Surgery |
| 9.2.2 | Ablation or Embolization Treatments |
| 9.2.3 | Radiation Therapy |
| 9.2.4 | Chemotherapy |
| 9.2.5 | Immunotherapy |
| 9.3 | Treatment of Hepatocellular Cancer |
| 9.3.1 | Surgery |
| 9.3.2 | Ablation |
| 9.3.3 | Embolization Therapy |
| 9.3.4 | Radiation Therapy |
| 9.3.5 | Targeted Drug Therapy |
| 9.3.6 | Immunotherapy |
| 9.3.7 | Chemotherapy |
| 9.4 | Treatment of Ovarian Cancer |
| 9.4.1 | Local Treatments |
| 9.4.1.1 | Surgery |
| 9.4.1.2 | Radiation Therapy |
| 9.4.2 | Systemic Treatment |
| 9.4.2.1 | Chemotherapy |
| 9.4.2.2 | Targeted Therapy |
| 9.4.2.3 | Hormone Therapy |
| 9.5 | Treatment for Colorectal Cancer |
| 9.5.1 | Local Treatments |
| 9.5.1.1 | Surgery |
| 9.5.1.2 | Radiation Therapy |
| 9.5.2 | Systemic Therapy |
| 9.5.2.1 | Chemotherapy |
| 9.5.2.2 | Targeted Therapy |
| 9.5.2.3 | Immunotherapy |
| 10 | Guidelines |
| 10.1 | NICE Guidelines for KRAS Mutation Testing of Tumors in Adults with Metastatic Colorectal Cancer (2013) |
| 10.1.1 | Diagnosis |
| 10.1.2 | Management/treatment |
| 10.1.2.1 | Chemotherapy |
| 10.1.2.2 | Biological Agents |
| 10.1.2.3 | Ongoing Care and Support |
| 10.1.3 | Patient Preferences and Issues |
| 10.1.4 | Scope of the Evaluation |
| 10.2 | ESMO Consensus Guidelines for the Management of Patients with Metastatic Colorectal Cancer (2016) |
| 10.2.1 | Recommendation for Tissue Selection |
| 10.2.2 | Recommendation for RAS testing |
| 10.2.3 | Recommendation for Conversion Therapy |
| 10.3 | NCCN Guidelines |
| 10.4 | Japanese Society of Medical Oncology Clinical Guidelines: Molecular Testing for Colorectal Cancer Treatment |
| 11 | Epidemiology and Patient Population |
| 11.1 | Key Findings |
| 11.2 | Assumptions and Rationale |
| 11.3 | KRAS Mutation in NSCLC |
| 11.3.1 | United States |
| 11.3.1.1 | Total Incident Cases of NSCLC in the United States |
| 11.3.1.2 | KRAS Incident Cases in NSCLC in the United States |
| 11.3.1.3 | KRAS Variant Cases in NSCLC in the United States |
| 11.3.2 | EU4 and the UK |
| 11.3.2.1 | Total Incident Cases of NSCLC in EU4 and the UK |
| 11.3.2.2 | KRAS Incident Cases in NSCLC in EU4 and the UK |
| 11.3.2.3 | KRAS Variant Cases in NSCLC in EU4 and the UK |
| 11.3.3 | Japan |
| 11.3.3.1 | Total Incident Cases of NSCLC in Japan |
| 11.3.3.2 | KRAS Incident Cases in NSCLC in Japan |
| 11.3.3.3 | KRAS Variant Cases in NSCLC in Japan |
| 11.4 | KRAS Mutation in CRC |
| 11.4.1 | United States |
| 11.4.1.1 | Total Incident Cases of CRC in the United States |
| 11.4.1.2 | KRAS Incident Cases in CRC in the United States |
| 11.4.1.3 | KRAS Variant Cases in CRC in the United States |
| 11.4.2 | EU4 and the UK |
| 11.4.2.1 | Total Incident Cases of CRC in EU4 and the UK |
| 11.4.2.2 | KRAS Incident Cases in CRC in EU4 and the UK |
| 11.4.2.3 | KRAS Variant Cases in CRC in EU4 and the UK |
| 11.4.3 | Japan |
| 11.4.3.1 | Total Incident Cases of CRC in Japan |
| 11.4.3.2 | KRAS Incident Cases in CRC in Japan |
| 11.4.3.3 | KRAS Variant Cases in CRC in Japan |
| 11.5 | KRAS Mutation in Pancreatic Cancer |
| 11.5.1 | United States |
| 11.5.1.1 | Total Incident Cases of Pancreatic Cancer in the United States |
| 11.5.1.2 | KRAS Incident Cases in Pancreatic Cancer in the United States |
| 11.5.1.3 | KRAS Variant Cases in Pancreatic Cancer in the United States |
| 11.5.2 | EU4 and the UK |
| 11.5.2.1 | Total Incident Cases of Pancreatic Cancer in EU4 and the UK |
| 11.5.2.2 | KRAS Incident Cases in Pancreatic Cancer in EU4 and the UK |
| 11.5.2.3 | KRAS Variant Cases in Pancreatic Cancer in EU4 and the UK |
| 11.5.3 | Japan |
| 11.5.3.1 | Total Incident Cases of Pancreatic Cancer in Japan |
| 11.5.3.2 | KRAS Incident Cases in Pancreatic Cancer in Japan |
| 11.5.3.3 | KRAS Variant Cases in Pancreatic Cancer in Japan |
| 12 | Marketed Drugs |
| 12.1 | Key Competitors |
| 12.2 | LUMAKRAS/LUMYKRAS (sotorasib): Amgen |
| 12.2.1 | Product Description |
| 12.2.2 | Regulatory Milestones |
| 12.2.3 | Other Developmental Activities |
| 12.2.4 | Ongoing Clinical Development |
| 12.2.5 | Safety and Efficacy |
| 12.2.6 | Product Profile |
| 12.3 | KRAZATI (adagrasib): Mirati Therapeutics |
| 12.3.1 | Product Description |
| 12.3.2 | Regulatory Milestones |
| 12.3.3 | Other Developmental Activities |
| 12.3.4 | Ongoing Clinical Development |
| 12.3.5 | Safety and Efficacy |
| 12.3.6 | Product Profile |
| 13 | Emerging Drugs |
| 13.1 | Key Competitors |
| 13.2 | JDQ443: Novartis |
| 13.2.1 | Product Description |
| 13.2.2 | Clinical Development |
| 13.2.2.1 | Clinical Trials Information |
| 13.2.3 | Safety and Efficacy |
| 13.3 | Divarasib: Roche/Genentech |
| 13.3.1 | Product Description |
| 13.3.2 | Other Development Activities |
| 13.3.3 | Clinical Development |
| 13.3.3.1 | Clinical Trials Information |
| 13.3.4 | Safety and Efficacy |
| 13.4 | Avutometinib (VS-6766): Verastem Oncology |
| 13.4.1 | Product Description |
| 13.4.2 | Other Developmental Activities |
| 13.4.3 | Clinical Development |
| 13.4.3.1 | Clinical Trials Information |
| 13.4.4 | Safety and Efficacy |
| 13.5 | RMC-4630: Revolution Medicines |
| 13.5.1 | Product Description |
| 13.5.2 | Other Development Activities |
| 13.5.3 | Clinical Development |
| 13.5.3.1 | Clinical Trials Information |
| 13.5.4 | Safety and Efficacy |
| 13.6 | Onvansertib: Cardiff Oncology |
| 13.6.1 | Product Description |
| 13.6.2 | Other Developmental Activities |
| 13.6.3 | Clinical Development |
| 13.6.3.1 | Clinical Trials Information |
| 13.6.4 | Safety and Efficacy |
| 13.7 | IMM-1-104: Immuneering Corporation |
| 13.7.1 | Product Description |
| 13.7.2 | Other Development Activities |
| 13.7.3 | Clinical Development |
| 13.7.3.1 | Clinical Trials Information |
| 13.7.4 | Safety and Efficacy |
| 13.8 | Glecirasib (JAB-21822): Jacobio Pharmaceuticals |
| 13.8.1 | Product Description |
| 13.8.2 | Other Development Activities |
| 13.8.3 | Clinical Development |
| 13.8.3.1 | Clinical Trials Information |
| 13.8.4 | Safety and Efficacy |
| 13.9 | BBP-398: BridgeBio Pharma (Navire Pharma) |
| 13.9.1 | Product Description |
| 13.9.2 | Other Development Activities |
| 13.9.3 | Clinical Development |
| 13.9.3.1 | Clinical Trials Information |
| 13.10 | MRTX1133: Mirati Therapeutics |
| 13.10.1 | Product Description |
| 13.10.2 | Other Development Activities |
| 13.10.3 | Clinical Development |
| 13.10.3.1 | Clinical Trials Information |
| 13.11 | DCC-3116: Deciphera Pharmaceuticals |
| 13.11.1 | Product Description |
| 13.11.2 | Other Development Activities |
| 13.11.3 | Clinical Development |
| 13.11.3.1 | Clinical Trials Information |
| 13.11.4 | Safety and Efficacy |
| 13.12 | ELI-002: Elicio Therapeutics |
| 13.12.1 | Product Description |
| 13.12.2 | Other Development Activities |
| 13.12.3 | Clinical Development |
| 13.12.3.1 | Clinical Trials Information |
| 13.13 | D-1553: InventisBio |
| 13.13.1 | Product Description |
| 13.13.2 | Other Development Activities |
| 13.13.3 | Clinical Development |
| 13.13.3.1 | Clinical Trials Information |
| 13.13.4 | Safety and Efficacy |
| 13.14 | SLATE-KRAS: Gritstone bio |
| 13.14.1 | Product Description |
| 13.14.2 | Other Development Activities |
| 13.14.3 | Clinical Development |
| 13.14.3.1 | Clinical Trials Information |
| 13.14.4 | Safety and Efficacy |
| 13.15 | D3S-001: D3 Bio |
| 13.15.1 | Product Description |
| 13.15.2 | Other Development Activities |
| 13.15.3 | Clinical Development |
| 13.15.3.1 | Clinical Trials Information |
| 14 | KRAS Inhibitors: The 7MM Analysis |
| 14.1 | Key Findings |
| 14.2 | Market Outlook |
| 14.3 | Key Market Forecast Assumptions |
| 14.4 | Total Market Size of KRAS-inhibitors in the 7MM |
| 14.5 | Market Size of KRAS-inhibitors by Therapies in the 7MM |
| 14.6 | United States |
| 14.6.1 | Total Market Size of KRAS-inhibitors in the United States |
| 14.6.2 | Market Size of KRAS-inhibitors by Therapies in the United States |
| 14.7 | EU4 and the UK |
| 14.7.1 | Total Market Size of KRAS-inhibitors in EU4 and the UK |
| 14.7.2 | Market Size of KRAS-inhibitors by Therapies in EU4 and the UK |
| 14.8 | Japan |
| 14.8.1 | Total Market Size of KRAS-inhibitors in Japan |
| 14.8.2 | Market Size of KRAS-inhibitors by Therapies in Japan |
| 15 | Unmet Needs |
| 16 | SWOT Analysis |
| 17 | KOL Views |
| 18 | Market Access and Reimbursement |
| 18.1 | United States |
| 18.1.1 | Centre for Medicare & Medicaid Services (CMS) |
| 18.2 | EU4 and the UK |
| 18.2.1 | Germany |
| 18.2.2 | France |
| 18.2.3 | Italy |
| 18.2.4 | Spain |
| 18.2.5 | United Kingdom |
| 18.3 | Japan |
| 18.3.1 | MHLW |
| 19 | KRAS-inhibitors Market Access and Reimbursement |
| 20 | Appendix |
| 20.1 | Bibliography |
| 20.2 | Report Methodology |
| 21 | DelveInsight Capabilities |
| 22 | Disclaimer |
Related Reports
Non-small Cell Lung Cancer Market
Non-small Cell Lung Cancer Market Insights, Epidemiology, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key NSCLC companies, including EMD Serono, Merck, Cellular Biomedicine Group, Inc., Celgene, CellSight Technologies, Inc., BeyondSpring Pharmaceuticals Inc., J Ints Bio, Forward Pharmaceuticals Co., Ltd., AstraZeneca, Bristol-Myers Squibb, Teligene US, Rain Oncology Inc, ReHeva Biosciences, Inc., Amgen, Novartis, RedCloud Bio, Parexel, Vitrac Therapeutics, LLC, Mythic Therapeutics, Instil Bio, Mirati Therapeutics Inc., Daiichi Sankyo, Inc., AstraZeneca, Precision Biologics, Inc, Promontory Therapeutics Inc., Palobiofarma SL, Regeneron Pharmaceuticals, Revolution Medicines, Inc., Cullinan Oncology, LLC, Iovance Biotherapeutics, Inc., Innate Pharma, among others.
Non-small Cell Lung Cancer Pipeline
Non-small Cell Lung Cancer Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key non-small cell lung cancer companies, including BridgeBio Pharma, Daiichi Sankyo, EMD Serono, Merck, BridgeBio Pharma, Abbvie, Pfizer, Eli Lilly and Company BioNTech SE, Shenzhen TargetRx, Taiho Pharmaceutical, Chong Kun Dang, Bristol Myers Squibb, Innovent Biologics, Xuanzhu Biopharmaceutical, Bayer, GeneScience Pharmaceuticals, InventisBio, Apollomics, Imugene, Ono Pharmaceutical, Pierre Fabre, Jiangsu Hengrui Medicine Co., Bristol-Myers Squibb, Surface Oncology, Inhibrx, Sinocelltech, Mirati Therapeutics, REVOLUTION Medicines, Yong Shun Technology Development, Iovance Biotherapeutics, Galecto Biotech, among others.
HER2-mutant Non-Small Cell Lung Cancer Pipeline
HER2-mutant Non-Small Cell Lung Cancer Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key HER2-mutant non-small cell lung cancer companies, including Dizal Pharmaceuticals, Puma Biotechnology, AstraZeneca, Jiangsu Hengrui Medicine, among others.
Small-cell Lung Cancer Pipeline
Small-cell Lung Cancer Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key small-cell lung cancer companies, including Ascentage Pharma, Merck & Co, AstraZeneca, Advenchen Laboratories, GlaxoSmithKline, Advanced Accelerator Applications, Trillium Therapeutics, Vernalis, Oncoceutics, NewBio Therapeutics, Wigen Biomedicine, Linton Pharm, Carrick Therapeutics, Xencor, Jiangsu HengRui Medicine, Aileron Therapeutics, Roche, Ipsen, Celgene, Lee's Pharmaceutical Limited, AbbVie, G1 Therapeutics, Chipscreen Biosciences, Luye Pharma Group, Shanghai Henlius Biotech, CSPC ZhongQi Pharmaceutical Technology, Impact Therapeutics, among others.
Small Cell Lung Cancer Market
Small Cell Lung Cancer Market Insights, Epidemiology, and Market Forecast – 2032 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key small cell lung cancer companies, including Ascentage Pharma, Merck & Co, AstraZeneca, Advenchen Laboratories, Advanced Accelerator Applications, Trillium Therapeutics, Wigen Biomedicine, Linton Pharm, Carrick Therapeutics, among others.
Oncology Conference Coverage Services
DelveInsight's Oncology Conference Coverage Services offer a thorough analysis of outcomes from major events like ASCO, ESMO, ASH, AACR, ASTRO, SOHO, SITC, the European CAR T-cell Meeting, and IASLC. This detailed examination provides businesses with essential insights for competitive intelligence and market trend forecasting, supporting the formulation of future strategies.
Other Business Consulting Services
Healthcare Competitive Intelligence
Healthcare Licensing Services
Healthcare Portfolio Management
Case Study
Learn how the engagement with respected KOLs bolstered the client's reputation as a leader in the pharma industry at KOL Profiling
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve .
Connect with us on LinkedIn | Facebook | Twitter
CONTACT: Contact Us Shruti Thakur ... +14699457679

Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.
















Comments
No comment