(MENAFN- Ameliorate Digital Consultancy)
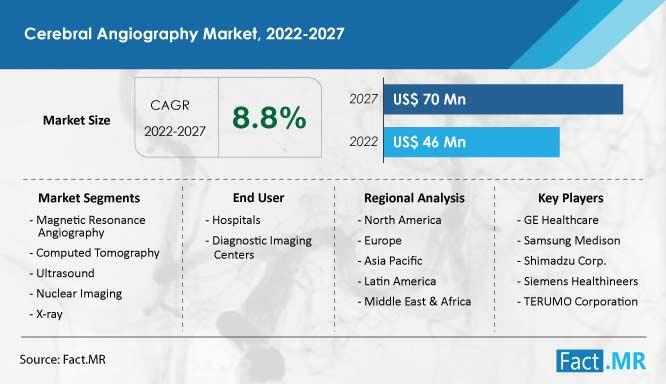
By the end of 2027, the cerebral angiography market , which is presently valued at US$ 46 million, is anticipated to have grown to US$ 70 million. From 2022 to 2027, the demand for cerebral angiography is expected to grow at a strong CAGR of 8.8%.
Using X-rays and contrast material containing iodine, a minimally invasive medical procedure called angiography produces images of the brain's blood vessels. Another name for cerebral angiography is intra-arterial digital subtraction angiography.
Download Sample Copy of This Report:
Market Titans
GE Healthcare Samsung Medison Shimadzu Corp. Siemens Healthineers TERUMO Corporation St. Jude Medical, Inc. Hitachi Medical Corporation Koninklijke Philips N.V. Toshiba Medical Systems Corporation
As vital diseases, including CVD, cancer, and neurological diseases become increasingly common, government organizations and healthcare companies around the globe are concentrating on improving their healthcare infrastructure as well as research and development for better diagnosis and treatment. These factors are driving the demand for cerebral angiography devices.
Growing cerebral angiography demand in hospitals is attributed to the availability of skilled and experienced medical professionals, employment of detecting technology in radiology departments to identify blood vessel damage brought on by trauma or injury, diagnose cardiovascular illnesses, including artery narrowing & detect aneurysms, and advancements in diagnostic technology.
Salient Points
Sales of cerebral angiography devices are expected to rise rapidly at a CAGR of 8.8% through 2027. The global cerebral angiography market is currently valued at US$ 46 million. Market in Japan is anticipated to expand at a CAGR of 5% over the forecast period. Market in Canada is set to progress at a CAGR of 8% through 2027.
Winning Strategy
Key companies are concentrating on product updates and new product development. Market players are seeking to develop cutting-edge, patient-focused products. By including a wide range of products in their portfolios, top industry players are concentrating on bolstering their positions in the market.
Key providers of cerebral angiography devices have been seen collaborating to maintain their market position as well as establish their geographic presence through effective distribution networks.
For instance,
The Discovery IGSTM 740 mobile angiography system from GE Healthcare was recently unveiled. Users can experience exceptional comfort and control with Discovery's rail-free design and versatile wide-bore C-arm configuration. Huge anatomies are also covered by its large detector in both 2D and 3D.
Companies in the cerebral angiography market are investing in R&D to diversify their product offerings. Partnerships between major industry players are anticipated to fuel market expansion over the coming years.
For instance :
In 2021, Royal Philips announced its interventional medicine vision by integrating its ground-breaking spectral CT imaging technology into a hybrid Angio CT suite. Through the integration of its distinctive Spectral CT 7500 system and its image-guided therapy system – Azurion with FlexArm into a single interventional suite solution, Philips wants to provide interventionalists with instant table-side access to these two important imaging modalities.
In-Depth Assessment on Key Segments
By Technology : Magnetic Resonance Angiography Computed Tomography Ultrasound Nuclear Imaging X-ray
By End User : Hospitals Diagnostic Imaging Centers Others
By Region : North America Europe Asia Pacific Latin America Middle East & Africa
Rapid Product Approval Processes and Increasing Angiography Device Usage in the U.S.
The market in the U.S. is expanding due to the widespread use of minimally-invasive treatments, the availability of reimbursements, rising adoption of cerebral thrombectomy devices, the growing elderly population, a strong network of medical device manufacturers, and the rising number of hospitals with lucrative funding prospects. Cardiovascular disease treatment with 3D printing is becoming more popular, and it may also be used to prevent disease in the future. Using heart valves that are 3D manufactured from living tissue, aortic valve failure can be addressed without the use of prosthetic valves. The market for cerebral angiography is being driven by healthcare industry innovation and investments.
The U.S. market is expanding due to rapid product approval processes and increasing angiography device usage as a result of the high volume of angiography screening procedures being conducted.
The global cerebral angiography report answers numerous pertinent questions, some of which are:
What are some of the latent areas of investments in the market? Which region is expected to emerge as showing the most attractive growth rate during the forecast period and which factors will be crucial to its growth? What trends are likely to change the status quo of the positions held by leading players of the market in the not-so-distant future? Which product/service/technology segments holds game-changing potential to dramatically shape the competitive dynamic in the market? What are the strategies adopted by top players to retain their stronghold in the market? Which strategic moves will new entrants adopt to gain a strong foothold in the market? What are COVID-19 implications on the market and learn how businesses can respond, manage, and mitigate the risks?
Get Customization on this Report for Specific Research Solutions:
Contact:
US Sales Office :
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Tel: +1 (628) 251-1583
E-Mail:




















Comments
No comment