(MENAFN- Netscribes) The COVID-19 pandemic, as well as the ensuing economic recession, have had a significant impact on many people’s mental health and created new challenges for those who already suffer from mental illness and substance use disorders.
According to a recent survey conducted by the Centers for disease Control and Prevention (CDC), 40.9% of the 5,470 respondents reported an adverse mental or behavioral health condition, such as anxiety, depression, or increased substance use. On the other hand, Kaiser Family Foundation research has also revealed that 53% of respondents believe that worrying about the continuing impact of Covid-19 is affecting their mental health.
Against this backdrop of a slow economic recovery and with no end in sight for the pandemic, the healthcare industry must focus on initiatives that address mental health disorders and develop new innovations to treat them. This is where digital therapeutics come into play.
How can DTx help? There have traditionally been two broad options for treating mental health and behavioral disorders: medicine and psychotherapy or behavioral therapies. Now doctors have a third option available at their fingertips: prompting behavioral changes in patients through the use of digital health devices , software, and applications. This form of treatment is known as “digital therapeutics (DTx).”
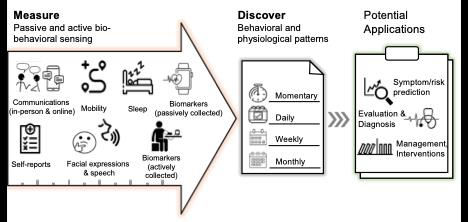
Recent technological advances have made it feasible to collect unobtrusive and efficient patient-generated health data from smartphones, which can be used to improve patient treatment and results. Smartphones, for instance, can collect data on sleep, activity, physiology, and device usage as well as provide context. DTx that uses digital phenotyping, “a moment-by-moment quantification of the individual's phenotype, using data from smartphones and other personal devices,” can take this a step further by giving recommended actions based on the phenotyping.
How digital phenotyping helps develop DTx programs
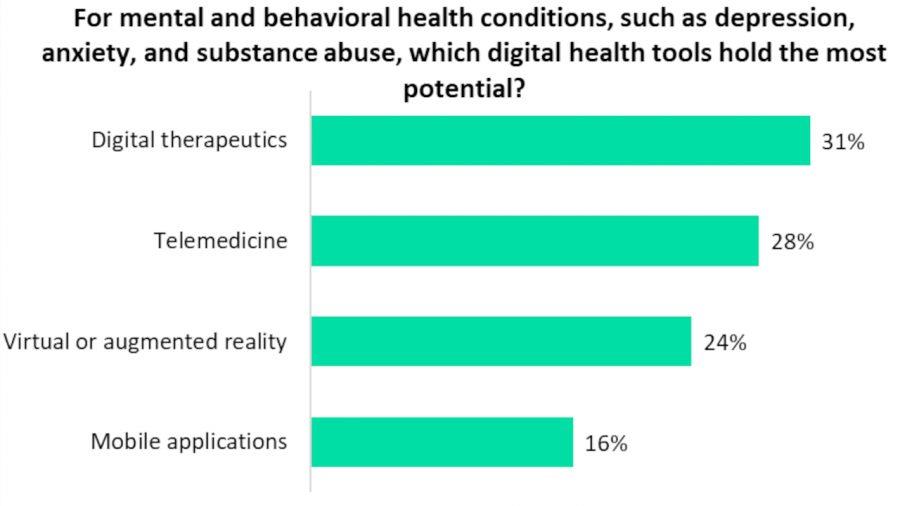
Source: Psychiatric Times The potential of DTx in mental health A recent study has found DTx to be the digital health tool with the most potential for treating mental and behavioral health conditions such as depression, anxiety, and substance abuse. It was voted as the top solution by a majority of 31% of survey respondents, which included both healthcare professionals, industry experts, and patients.
Source: Pharmaceutical technology DTx in mental health has a high likelihood to reduce treatment gaps in mental health. Over 85% of people with mental disorders currently use mobile phones as part of their daily routines, and more than 60% own smartphones. Many people with such conditions have indicated an interest in learning how to utilize mobile apps to regulate their emotions, monitor mental health symptoms, and obtain digital therapies.
Some DTx are intended to be used in conjunction with medications or behavioral therapy. Others aim to completely replace conventional treatments. Web-based cognitive behavioral therapy (CBT) programs, digital dashboards that allow patients and doctors to carefully monitor health indicators, and even game-based interventions are some of the most prominent examples.
Benefits of using DTx in mental health

Source: Psychiatric Times For instance, Big Health , a digital behavioral health startup, has developed Sleepio , an evidence-based DTx platform that uses CBT for treating insomnia. Instead of being delivered in a psychologist’s office, Sleepio’s six-week intervention is conducted by an animated virtual sleep specialist named The Prof and his narcoleptic dog Pavlov. “It’s supposed to feel enjoyable, but it’s actually high-octane CBT,” explains Dr. Colin Espie, Chief Medical Officer of Big Health.
Meanwhile, other companies are developing solutions that will be used jointly by patients and their healthcare providers. For example, Pear Therapeutics , a Boston and San Francisco-based startup, is developing prescription-based DTx for substance use disorder, post-traumatic stress disorder, generalized anxiety disorder, and schizophrenia.
Partnerships and M & As Partnerships, according to experts , are a successful route to market for DTx in mental health solutions. However, while the use of DTx in this field is a relatively new option, there are some key challenges in deploying it, such as low reimbursement rates, the development of considerable evidence to support its therapeutic value, patient participation, and, lastly, physician time and knowledge.
Despite all odds, numerous firms across the healthcare sector have recognized the potential of the DTx in the mental health market and are becoming more involved in this field through collaborations and even M & As. For instance, in the pharmaceutical sector, Boehringer Ingelheim has struck a USD 500M partnership deal with prescription DTx firm Click Therapeutics to co-develop and commercialize digital treatments for schizophrenia patients. Med-tech company Jolly Good Inc. and Teijin Pharma Ltd., on the other hand, have also inked a commercial cooperation agreement to develop a VR digital therapeutics solution for treating Major Depressive Disorder (MDD).
Meanwhile, in the digital health and virtual care space, Pear Therapeutics has teamed with Sandoz, a Novartis business, to promote the introduction of reSET , a treatment for substance abuse disorder, which is its first FDA-approved prescription DTx. Meanwhile, Sanofi has also teamed up with Happify Health to develop a prescription DTx for depression treatment in people with multiple sclerosis.
As for M & As, the healthcare industry witnessed a record-breaking deal in Q3 2020, worth USD 18.5B, when telehealth giant Teladoc merged with DTx-based chronic disease management platform Livongo. This merger will help combine Livongo's DTx expertise with Teladoc's teletherapy solutions . To add to the list, Amwell, a telehealth giant, has recently acquired SilverCloud Health, a DTx-based mental health platform. This acquisition will allow Amwell to deliver care in between one-time virtual visits, by using interactive DTx treatments.
The regulatory scenario of DTx in mental health As the use of DTx in mental health gains momentum, the FDA has been tasked with determining how to effectively evaluate them. For regulatory organizations, pathways for medical devices include software that is designed to diagnose or treat medical diseases, also known as software-as-a- medical-device (SaMD). However, because such software is comparatively different from existing devices, the approval process is not simple.
Recognizing that SaMDs necessitate updates differently than traditional pharmaceuticals, the FDA has been working on a precertification approach in which individual companies, rather than specific interventions, can receive approval based on the quality of their development process. The program recognizes developers who can monitor real-world efficacy and takes into account that software products benefit from insight gained after being released for widespread use.
To date, nine companies have signed up for the precertification program, and early indications show that it will result in a simplified development pathway for DTx. Regulatory frameworks comparable to the FDA’s precertification program, if effective, may aid in bringing together development pathways that combine both rapid pace and rigorous research.
Given the pandemic’s impact on mental health, the FDA has also temporarily eased its regulatory stance on computerized behavioral therapy for psychiatric disorders, which is further aiding the widespread adoption of DTx in mental health by HCPs across the globe.
The road ahead Healthcare delivery will continue to be dominated by in-person therapy, teletherapy, and pharmaceuticals. However, it has become clear that these measures are insufficient to solve the current mental health crisis in the United States and other countries. The enormous growth of the digital health field during the pandemic has supported DTx in finding ready markets for its products, but the emerging sector faces regulatory and reimbursement challenges.
At a time when healthcare demand is unprecedented, DTx adoption is unavoidable as implementing more digital solutions allows for a greater reach than in-person care alone. While regulatory and insurance issues must yet be worked out, digital medicine pioneers believe it is only a matter of time until physicians begin prescribing DTx regularly.
Netscribes offers holistic market, consumer, and technology insights for healthcare organizations to help them navigate the changes in the industry. Contact us to know more.
MENAFN19102021005964013036ID1103000707