(MENAFN- PR Newswire)
Code paves the way for reimbursement and expanded clinical use of AI-enabled heart disease detection
SAN FRANCISCO, Nov. 14, 2024 /PRNewswire/ --
Eko health , a pioneer in applying artificial intelligence for early detection of heart and lung diseases, today announced the issuance of a Category III Current Procedural Terminology (CPT) code by the American Medical Association (AMA) for its SENSORATM platform. The newly issued Category III CPT code will be effective on July 1, 2025, and is the first step to coverage and reimbursement for SENSORATM, bringing advanced heart disease detection to clinicians across the U.S.
Continue Reading
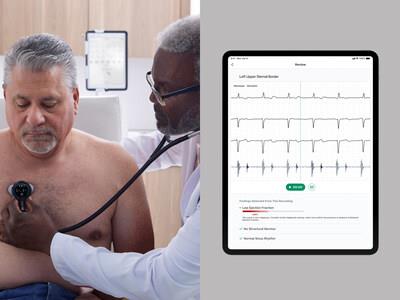
Code paves the way for reimbursement and expanded clinical use of AI-enabled heart disease detection.
SENSORA is an advanced early disease detection platform that includes FDA-cleared AI algorithms that identify signs of structural heart murmurs, low ejection fraction (Low EF), and arrhythmias, including AFib, in the front-line care setting. It seamlessly integrates with Eko's CORE 500TM digital stethoscope, which embeds advanced ECG technology in a tool that every provider is familiar with.
Heart disease remains the leading cause of death in the United States, accounting for about 1 in every 5 deaths, according to the CDC. Early detection plays a critical role in improving patient outcomes. By empowering primary care providers to identify cardiac issues earlier, SENSORATM enhances proactive care and reduces the burden of advanced heart disease management.
"The AMA's creation of Category III CPT code for Eko's AI disease detection algorithms is a major step in increasing access to early heart disease detection," said Connor Landgraf, CEO of Eko Health. "This milestone will help enable clinicians to use powerful, validated tools to identify heart disease early, ultimately improving patient outcomes, especially in communities with limited access to specialist care."
SENSORA's AI algorithms, validated in clinical and real-world studies, assist clinicians in detecting cardiac disease signs often missed by standard care. An independent clinical validation published in Circulation
showed that Eko's structural heart murmur detection AI doubled sensitivity for structural heart disease compared to an analog stethoscope. Additionally, a Lancet Digital Health
study involving over 1,000 patients confirmed Eko's low ejection fraction AI accurately detects reduced ejection fraction in primary care, leading to its deployment in over 100 UK clinics by the NHS and Imperial College London.
The Category III CPT code reflects SENSORA's growing role as an essential tool in cardiac care and is expected to expand its use across multiple clinical environments. This designation is a testament to Eko's commitment to equipping primary care clinicians with AI tools that elevate patient care standards and bridge the preventive care gap.
About Eko Health
Eko Health is a leading digital health company advancing how healthcare professionals detect and monitor heart and lung disease with its portfolio of digital stethoscopes, patient and provider software, and AI-powered analysis. Its FDA-cleared platform, used by over 500,000 healthcare professionals worldwide, allows them to detect earlier and with higher accuracy, diagnose with more confidence, manage treatment effectively, and ultimately give their patients the best care possible. Eko Health is headquartered in Emeryville, California, with over $165 million in funding from ARTIS Ventures, DigiTx Partners, Double Point Ventures, EDBI, Highland Capital Partners, LG Technology Ventures, Mayo Clinic, Morningside Technology Ventures Limited, NTTVC, Questa Capital, and others.
For more information, visit .
Media Contact
Sam Moore
[email protected]
SOURCE Eko Health
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE?
440k+
Newsrooms &
Influencers
9k+
Digital Media
Outlets
270k+
Journalists
Opted In
GET STARTED
MENAFN14112024003732001241ID1108886131
Legal Disclaimer:
MENAFN provides the information “as is” without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the provider above.