(MENAFN- GlobeNewsWire - Nasdaq) The dynamics of the non-melanoma skin cancer market are anticipated to change in the coming years due to the improvement in the rise in healthcare spending across the world. Key players, such as Sirnaomics, AiViva BioPharma, Replimune, and others, are developing drugs for NMSC.New York, USA, Oct. 23, 2024 (GLOBE NEWSWIRE) -- Non-melanoma Skin Cancer is Predicted to Exhibit Remarkable Growth During the Study Period (2020–2034) | DelveInsight
The dynamics of the non-melanoma skin cancer market are anticipated to change in the coming years due to the improvement in the rise in healthcare spending across the world. Key players, such as Sirnaomics, AiViva BioPharma, Replimune, and others, are developing drugs for NMSC.
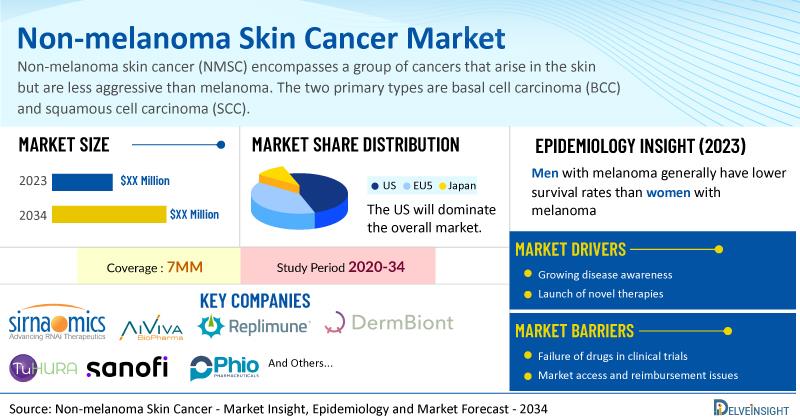
DelveInsight's Non-melanoma Skin Cancer Market Insights report includes a comprehensive understanding of current treatment practices, emerging non-melanoma skin cancer drugs, market share of individual therapies, and current and forecasted non-melanoma skin cancer market size from 2020 to 2034, segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan].
Key Takeaways from the Non-melanoma Skin Cancer Market Report
According to DelveInsight's analysis, the market size of non-melanoma skin cancer in the 7MM is expected to grow at a significant CAGR by 2034. As per DelveInsight's estimate, men with melanoma generally have lower survival rates than women with melanoma. In the United States, around 3.6 million cases of BCC and 1.8 million cases of SCC are diagnosed annually, with the incidence increasing with age. Prominent companies working in the domain of non-melanoma skin cancer, including Sirnaomics, AiViva Biopharma, Replimune, DermBiont, Inc., TuHURA Biosciences, Inc., Sanofi, Phio Pharmaceuticals Inc. , and others, are actively working on innovative non-melanoma skin cancer drugs. These novel non-melanoma skin cancer therapies are anticipated to enter the non-melanoma skin cancer market in the forecast period and are expected to change the market. Some of the key non-melanoma skin cancer treatments include STP705, AIV001, RP1, SM-020 1% Gel, IFx-Hu2.0, THOR-707, PH-762 , and others.
Discover which therapies are expected to grab the non-melanoma skin cancer market share @ Non-melanoma Skin Cancer Market Report
Non-melanoma Skin Cancer Overview
Non-melanoma skin cancer (NMSC) encompasses a group of cancers that arise in the skin but are less aggressive than melanoma. The two primary types are basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). NMSC is the most common form of skin cancer worldwide. The primary cause of NMSC is ultraviolet radiation from the sun or tanning beds, which damages the DNA in skin cells over time. Other factors that increase risk include fair skin, a history of sunburns, chronic exposure to sunlight, a weakened immune system, and exposure to harmful substances such as arsenic.
NMSC typically presents as an abnormal skin lesion that may vary in appearance. Basal cell carcinoma often appears as a pearly or waxy bump, flat scar-like lesion, or small blood vessel–filled patch. Squamous cell carcinoma may present as a firm, red nodule, or a flat lesion with a scaly, crusted surface. Lesions may bleed, not heal properly, or grow over time.
Diagnosis of NMSC starts with a visual examination by a dermatologist. Suspicious lesions are biopsied-small tissue samples are taken for microscopic examination. Other diagnostic tools, such as dermoscopy, may be used to assess the lesion in detail. Early detection is crucial, as NMSC can often be treated effectively, especially if caught early.
Non-melanoma Skin Cancer Epidemiology Segmentation
The non-melanoma skin cancer epidemiology section provides insights into the historical and current non-melanoma skin cancer patient pool and forecasted trends for the 7MM. It helps recognize the causes of current and forecasted patient trends by exploring numerous studies and views of key opinion leaders.
The non-melanoma skin cancer market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM segmented into:
Total Prevalent Cases of NMSC Total Diagnosed Cases of NMSC Gender-specific Cases of NMSC Region-specific Cases of NMSC Age-specific Cases of NMSC Treatable Cases of NMSC
Download the report to understand which factors are driving non-melanoma skin cancer epidemiology trends @ Non-melanoma Skin Cancer Epidemiological Insights
Non-melanoma Skin Cancer Treatment Market
Treatment options for non-melanoma skin cancer depend on various factors, such as the type, size, and aggressiveness of the tumor, as well as whether it has spread. Surgery is typically the main approach, to completely remove the tumor. During surgery, the tumor and some surrounding tissue are excised to ensure no cancer cells are left behind, reducing the risk of recurrence. Smaller surgical wounds may heal with minimal scarring, but larger procedures can result in noticeable marks.
In February 2021, the US FDA approved LIBTAYO (cemiplimab-rwlc) , a PD-1 inhibitor, as the first immunotherapy for advanced basal cell carcinoma in patients who had previously been treated with a hedgehog pathway inhibitor or for whom HHI therapy was unsuitable. The approval included full authorization for treating locally advanced BCC and accelerated approval for metastatic BCC.
Although there are no current FDA approvals for using KEYTRUDA as a first-line therapy for locally advanced or metastatic basal cell carcinoma, it is approved for advanced cutaneous squamous cell carcinoma. On June 24, 2020, the FDA approved pembrolizumab (KEYTRUDA) for patients with recurrent or metastatic CSCC that cannot be treated with surgery or radiation. In July 2015, Novartis announced the FDA approval of ODOMZO (sonidegib, previously LDE225) 200 mg capsules for treating adults with locally advanced BCC. Additionally, in August 2015, the European Commission approved ODOMZO.
Learn more about the market of non-melanoma skin cancer @ Non-melanoma Skin Cancer Treatment
Non-melanoma Skin Cancer Emerging Drugs and Companies
A few emerging therapies like STP705 (Sirnaomics), AIV001 (AiViva Biopharma), RP1 (Replimune), and others, are being developed in late-stage clinical development.
Sirnaomics' product candidate, STP705 , is a small interfering RNA (siRNA) therapy that utilizes the company's proprietary dual-target inhibitory mechanism and polypeptide nanoparticle (PNP) delivery system to effectively reduce the expression of the TGF-β1 and COX-2 genes. These targets are well-established as critical in the development of drugs for oncology and fibrosis diseases. Currently, STP705 is focused on three key programs: advancing late-stage clinical trials for squamous cell carcinoma in situ (isSCC), completing a Phase II trial for basal cell carcinoma, and initiating a Phase I trial for fat remodeling.
AIV001 is a new formulation that combines a multi-kinase inhibitor with AiViva's unique delivery technology, aimed at achieving extended drug release through intradermal application. It targets various pathways to decrease fibroplasia during different stages of wound healing and scarring, inhibits VEGFR to reduce inflammation and fibrosis linked to rosacea, and mitigates neovascularization and cell proliferation associated with specific cancers. In nonclinical in vivo studies, AIV001 showed a reduction in dermal neovascularization and fibroplasia in wound healing models, along with a prolonged presence in the skin from a single application. AiViva has successfully completed a Phase I/IIa clinical trial of AIV001 focused on incisional scarring and wound healing, as well as a clinical trial for non-melanoma skin cancer.
Replimune's RP1 aims to target tumor types that are more responsive to the immune system. The GALV-GP R- protein it encodes boosts the virus's ability to kill tumors and promotes immunogenic cell death. A Phase II clinical trial is currently underway to evaluate the investigational oncolytic immunotherapy RP1 in combination with nivolumab in adult patients with advanced or refractory solid tumors, including non-melanoma skin cancer.
The other pipeline therapies for non-melanoma skin cancer include
SM-020 1% Gel: DermBiont, Inc. IFx-Hu2.0: TuHURA Biosciences, Inc. THOR-707: Sanofi PH-762: Phio Pharmaceuticals Inc.
The anticipated launch of these emerging therapies are poised to transform the non-melanoma skin cancer market landscape in the coming years. As these cutting-edge therapies continue to mature and gain regulatory approval, they are expected to reshape the non-melanoma skin cancer market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about non-melanoma skin cancer clinical trials, visit @ Non-melanoma Skin Cancer Treatment Drugs
Non-melanoma Skin Cancer Market Dynamics
The non-melanoma skin cancer market dynamics are anticipated to change in the coming years. The rising incidence of NMSC globally , driven by factors such as aging populations, increased UV radiation exposure , and improved diagnostic tools , is a key strength propelling the NMSC market growth, while abundant opportunities in the NMSC treatment market stem from both the increasing global incidence rates and the development of advanced treatment modalities .
Furthermore, many potential therapies are being investigated for the treatment of non-melanoma skin cancer, and it is safe to predict that the treatment space will significantly impact the non-melanoma skin cancer market during the forecast period. Moreover, the anticipated introduction of emerging therapies with improved efficacy and a further improvement in the diagnosis rate is expected to drive the growth of the non-melanoma skin cancer market in the 7MM.
However, several factors may impede the growth of the non-melanoma skin cancer market. The lack of drugs in the pipeline for NMSC treatment, due to minimal research and development efforts focused on new therapies, along with serious side effects of treatment options like radiation therapy-such as edema, skin peeling, skin problems, and hair loss-can restrict its adoption and impede market growth and innovation in treatment options for patients suffering from this common type of skin cancer.
Moreover, non-melanoma skin cancer treatment poses a significant economic burden and disrupts patients' overall well-being and QOL. Furthermore, the non-melanoma skin cancer market growth may be offset by failures and discontinuation of emerging therapies , unaffordable pricing , market access and reimbursement issues , and a shortage of healthcare specialists . In addition, the undiagnosed, unreported cases and the unawareness about the disease may also impact the non-melanoma skin cancer market growth.
| Non-melanoma Skin Cancer Report Metrics | Details |
| Study Period | 2020–2034 |
| Non-melanoma Skin Cancer Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Key Non-melanoma Skin Cancer Companies | Sirnaomics, AiViva Biopharma, Replimune, DermBiont, Inc., TuHURA Biosciences, Inc., Sanofi, Phio Pharmaceuticals Inc., and others |
| Key Non-melanoma Skin Cancer | STP705, AIV001, RP1, SM-020 1% Gel, IFx-Hu2.0, THOR-707, PH-762, and others |
Scope of the Non-melanoma Skin Cancer Market Report
Non-melanoma Skin Cancer Therapeutic Assessment: Non-melanoma Skin Cancer current marketed and emerging therapies Non-melanoma Skin Cancer Market Dynamics: Conjoint Analysis of Emerging Non-melanoma Skin Cancer Drugs Competitive Intelligence Analysis: SWOT analysis and Market entry strategies Unmet Needs, KOL's views, Analyst's views, Non-melanoma Skin Cancer Market Access and Reimbursement
Discover more about non-melanoma skin cancer in development @ Non-melanoma Skin Cancer Clinical Trials
Table of Contents
| 1. | Non-melanoma Skin Cancer Market Key Insights |
| 2. | Non-melanoma Skin Cancer Market Report Introduction |
| 3. | Non-melanoma Skin Cancer Market Overview at a Glance |
| 4. | Non-melanoma Skin Cancer Market Executive Summary |
| 5. | Disease Background and Overview |
| 6. | Non-melanoma Skin Cancer Treatment and Management |
| 7. | Non-melanoma Skin Cancer Epidemiology and Patient Population |
| 8. | Patient Journey |
| 9. | Non-melanoma Skin Cancer Marketed Drugs |
| 10. | Non-melanoma Skin Cancer Emerging Drugs |
| 11. | Seven Major Non-melanoma Skin Cancer Market Analysis |
| 12. | Non-melanoma Skin Cancer Market Outlook |
| 13. | Potential of Current and Emerging Therapies |
| 14. | KOL Views |
| 15. | Unmet Needs |
| 16. | SWOT Analysis |
| 17. | Appendix |
| 18. | DelveInsight Capabilities |
| 19. | Disclaimer |
| 20. | About DelveInsight |
Related Reports
Non-melanoma Skin Cancer Epidemiology Forecast
Non-melanoma Skin Cancer Epidemiology Forecast – 2032 report delivers an in-depth understanding of the disease, historical and forecasted non-melanoma skin cancer epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Non-melanoma Skin Cancer Pipeline
Non-melanoma Skin Cancer Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key non-melanoma skin cancer companies, including Sirnaomics, AiViva Biopharma, Replimune, DermBiont, Inc., TuHURA Biosciences, Inc., Sanofi, Phio Pharmaceuticals Inc., among others.
Basal Cell Carcinoma Market
Basal Cell Carcinoma Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key basal cell carcinoma companies including MedC Biopharma Corporation, AiViva BioPharma, MediWound, Kintara Therapeutics, IO Biotech, Sirnaomics, Aresus Pharma, Epitome Pharmaceuticals, Transgene, Senhwa Biosciences, Palvella Therapeutics, Suzhou Kintor Pharmaceuticals, Leaf Vertical, among others.
Basal Cell Carcinoma Pipeline
Basal Cell Carcinoma Pipeline Insight – 2024 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key basal cell carcinoma companies, including PellePharm, MedC Biopharma Corporation, AiViva BioPharma, MediWound, Kintara Therapeutics, IO Biotech, Sirnaomics, Aresus Pharma, Epitome Pharmaceuticals, Transgene, Senhwa Biosciences, Palvella Therapeutics, Suzhou Kintor Pharmaceuticals, Leaf Vertical, among others.
Cutaneous Squamous Cell Carcinoma Market
Cutaneous Squamous Cell Carcinoma Market Insights, Epidemiology, and Market Forecast – 2034 report deliver an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key cutaneous squamous cell carcinoma companies including Incyte Corporation, Shanghai Henlius Biotech, Novartis, Rakuten Medical, Morphogenesis, Genentech, Berg Pharma, I-MAB Biopharma, Roche, Genexine, CureVac, among others.
Best-in-class Business Consulting Services
Healthcare Conference Coverage
Healthcare Competitive Intelligence
Healthcare Licensing Services
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve .
Connect with us on LinkedIn | Facebook | Twitter
CONTACT: Contact Us
Shruti Thakur
...
+14699457679

MENAFN23102024004107003653ID1108812130





















Comments
No comment