(MENAFN- Ameliorate Digital Consultancy)
Wearable technology is bound to come up as one of the prospective solutions for ascertaining assistive healthcare services for everyone. The common applications are inclusive of managing several health conditions through wearable devices, preventive care to ICU and elderly patients, and offering quality remote care to various patients at remote areas or care homes, and likewise. This would be the conduct of Animal Health Active Pharmaceutical Ingredients market in the forecast period.
The global animal health active pharmaceutical ingredient (API) market is projected to register an impressive expansion at 7.3% CAGR during the forecast period 2017 to 2025, according to a recent study by Persistence Market Research (PMR). PMR's study estimates the market to increase from revenues worth US$ 5,216.1 Mn in 2017 to reach US$ 9,162.2 Mn by 2025-end.
Access Free Sample Copy Of Animal Health Active Pharmaceutical Ingredients Market Report@
Company Profiles
Glenmark Pharmaceutical Ltd. Zoetis Inc. Eli Lilly & Co. Sanofi Winthrop Industry (CEPiA) Indukern, SA Ofichem BV P&R SpA (Olon SpA) Lonza Group AG Huvepharma fine gold animal health Blanver Farmoquimica E Farmaceutica SA Zhejiang Hisun Pharmaceutical Co. Ltd Changzhou Yabang-Qh Pharmachem Co., Ltd. Shanghai Pharmtech Co. Ltd. Ningxia Tairui Pharma CO. Ltd. Shaanxi Hanjiang Pharmaceutical Group Co.,Ltd. Sequent Scientific Ltd. NGL Fine Chem Ltd. Omkar Speciality Chemicals Limited Excel Industries Ltd. Others
How About Revitalizing The Strategy-Oriented Funnel To Stay Ahead In The Animal Health Active Pharmaceutical Ingredients Market@
Limited Insurance or Government Intervention Provides Smooth Flow to the Market Growth in APAC
Asia Pacific (APAC) will continue to be the most lucrative region in the global animal health active pharmaceutical ingredient market, with sales exhibiting the second highest CAGR through 2025. APAC has remained one of the largest API suppliers, at affordable costs.
Governments across APAC have been making investments and focusing on local manufacturing facilities of APIs. Favorable policies offered by these governments have been shifting the focus of API manufacturers in moving their production bases to APAC countries, such as India and China.
In addition, limited insurance or government intervention provides smooth flow to market growth in these regions.
However, animal health API products manufactured in APAC countries do not meet regulatory standards, and there has been increasing concerns pertaining to the quality of APIs, especially those produced in China and India. Western regulatory bodies are becoming stricter in the inspection of APIs produced in these regions.
Manufacturers are further facing challenges in bridging the gap between the cost and quality of APIs. These factors will inhibit growth of the market in APAC.
Europe to Remain the Second Largest Market for Animal Health APIs
Europe will remain the second largest market for animal health active pharmaceutical ingredients. Leading healthcare companies are located in the Europe, which in turn has boosted requirement for veterinary APIs in the region. Consumption of veterinary drugs in Europe has surged substantially over the past few years. Spain has been witnessed to be the largest consumer of veterinary antimicrobial APIs in the EU.
In addition, rising improvements in the animal productivity has driven growth of the animal health industry in this region. These incidences are expected to augment growth of the animal health API market in Europe.
However, the pharmaceutical industry in Europe has been concerned with potential short-term impacts of REACH regulations. This is likely to result in decreased supply of materials, or reagents, which are not preregistered by EU suppliers.
In addition, dynamic changes in Europe's macro-economic environment, such as the Brexit, has been impacting the intra-European trade and overall pharmaceutical sector. These factors might curb growth of the animal health API market in Europe.
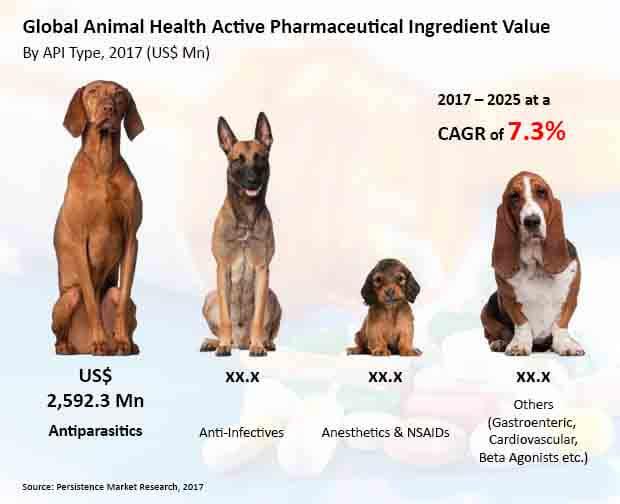
Antiparasitics to Remain the Largest Adopted APIs in the Market
Antiparasitics will continue to be the largest adopted API in the market, followed by anti-infectives. Sales of Antiparasitics are expected to register the fastest expansion, to account for the largest revenues by 2025-end. In contrast, revenues from sales of anesthetics and NSAIDs will remain comparatively lower than antiparasitics and anti-infectives.
Region Coverage (Regional Production, Demand & Forecast by Countries etc.):
North America (U.S., Canada, Mexico)
Europe (Germany, U.K., France, Italy, Russia, Spain etc.)
Asia-Pacific (China, India, Japan, Southeast Asia etc.)
South America (Brazil, Argentina etc.)
Middle East & Africa (Saudi Araia, South Africa etc.)
Go To“Purchase Now” To Have Our Animal Health Active Pharmaceutical Ingredients Market Report!
Key Questions Answered in This Report.
What will the Market growth rate in Future? What are the key factors driving the global Market? Who are the key manufacturers in Market space? What are the opportunities and threats faced by the vendors in the global industry? What are sales, revenue, and price analysis by regions of industry?
About Us:
Persistence Market Research (PMR), as a 3rd-party research organization, does operate through an exclusive amalgamation of market research and data analytics for helping businesses ride high, irrespective of the turbulence faced on the account of financial/natural crunches.
Contact Us:
Persistence Market Research
Address – 305 Broadway, 7th Floor, New York City, NY 10007 United States
U.S. Ph. – +1-646-568-7751
USA-Canada Toll-free – +1 800-961-0353
Sales –