(MENAFN- PR Newswire)
MIDDLETON, Wis., Oct. 10, 2024 /PRNewswire/ -- Natus Medical Incorporated announced it has submitted an FDA 510(k) premarket notification to the U.S. Food and Drug Administration (FDA) for its Natus point-of-care EEG device.
The rapid deploy decision-support tool is designed to quickly help identify non-convulsive seizures (NCSs) and status epilepticus in acute care environments, supporting rapid intervention and streamlining treatment decisions to improve patient outcomes.
Continue Reading
Clinicians involved in the system's evaluation expressed high expectations for a point-of-care EEG solution from Natus
Post this
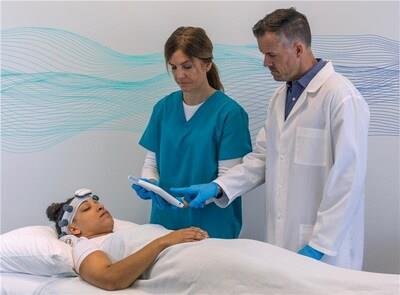
The highly anticipated point-of-care EEG system compliments the robust and reliable Natus portfolio of ICU solutions
This point-of-care EEG system leverages Natus' leading NeuroWorks software platform to provide a seamless and familiar review experience with remote neurologist collaboration, 24/7 from any web browser. NeuroWorks EEG systems are trusted by more health systems and small practices around the world. With its unparalleled expertise in complex IT environments, Natus will utilize a leading cybersecurity-certified cloud platform to ensure patient data security.
Natus has partnered with Persyst, the most widely accepted developer of its gold standard spike and seizure detection, to incorporate AI-powered seizure detection algorithms, also under review for FDA 510(k) clearance.
Clinicians involved in the system's evaluation expressed high expectations for a point-of-care EEG solution from Natus as the leading provider of EEG neurodiagnostic solutions. Early feedback promotes an easy-to-use system that can be deployed in minutes by ER and ICU staff without the need for an EEG technologist and with clinical information exceeding the expectations of neurologists.
Backed by decades of EEG leadership, Natus point-of-care EEG is anticipated to deliver the confidence and precision care providers need to support making informed, life-saving decisions in the emergency department and ICU. Natus, the brand trusted by more neurologists worldwide, aspires to set a new standard for reliable EEG in critical care moments.
ABOUT
NATUS
Natus is trusted by healthcare providers around the globe as the solution source to screen, diagnose, and treat disorders affecting the brain, neural pathways, and sensory nervous systems. Natus' best-in-class solutions, including service, field support and
education , enable clinicians to advance their standard of care, improving patient outcomes and quality of life.
CONTACT:
Lisa Schuler
Sr. Director, Corporate Communications
Phone: +1 612 528-1332
Email: [email protected]
natus
SOURCE NATUS MEDICAL INCORPORATED
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE?
440k+
Newsrooms &
Influencers
9k+
Digital Media
Outlets
270k+
Journalists
Opted In
GET STARTED
MENAFN10102024003732001241ID1108768323
Legal Disclaimer:
MENAFN provides the information “as is” without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the provider above.