
Coronavirus Test Kits Market In North America To Shrink By USD 1.41 Billion (2024-2028) As Integration With Routine Healthcare Evolves, AI-Powered Report By Technavio
| Coronavirus Test Kits Market In North America Scope |
|
| Report Coverage |
Details |
| Base year |
2023 |
| Historic period |
2018 - 2022 |
| Forecast period |
2024-2028 |
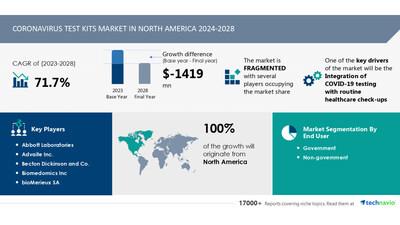
| Growth momentum & CAGR |
Decelerate at a CAGR of -71.7% |
| Market growth 2024-2028 |
USD -1419 million |
| Market structure |
Fragmented |
| YoY growth 2022-2023 (%) |
-88.9 |
| Regional analysis |
North America |
| Performing market contribution |
North America at 100% |
| Key countries |
US, Canada, and Mexico |
| Key companies profiled |
Abbott Laboratories, Advaite Inc., Becton Dickinson and Co., Biomedomics Inc, bioMerieux SA, Cellex Inc., Co Diagnostics Inc, Cue Health Inc., Danaher Corp., DiaSorin SpA, F. Hoffmann La Roche Ltd., Hologic Inc., Laboratory Corp. Of America Holdings, Mammoth Biosciences Inc., MyBioSource Inc., QIAGEN N.V., QuidelOrtho Corp., RayBiotech Life Inc., Robert Bosch GmbH, Sherlock Biosciences, Talis Biomedical Corp, and Thermo Fisher Scientific Inc. |
Market Driver
The coronavirus test kits market in North America is experiencing significant growth due to the ongoing research and development of COVID-19 vaccines and tests. Product quality and visibility are crucial factors driving sales. Vendors are promoting test kits through websites and awareness programs. Notably, several research organizations are developing multi-sample testing kits. The US Food and Drug Administration (FDA) granted an Emergency Use Authorization (EUA) to Lucira Health for the first over-the-counter (OTC) at-home diagnostic test that identifies both influenza A and B and SARS-CoV-2 in around 30 minutes. In addition, Talis Biomedical launched the Rapid Point-of-Care COVID-19 Test kit, which can identify SARS-CoV-2 in under 30 minutes using a compact instrument. Thermo Fisher Scientific introduced a new COVID-19 test kit that identifies the presence of any one of eight SARS-CoV-2 gene targets, providing reliable results even for potential virus mutations. These new test kit launches positively impacted the growth of the coronavirus test kits market in North America. However, market growth is dependent on the occurrence of future COVID-19 events.
The Coronavirus market in North America is currently focused on RT PCR tests for COVID-19 diagnosis. With the ongoing pandemic, there is a high demand for COVID-19 Test Kits using various diagnostic techniques. Immunization coverage and booster doses are also being closely monitored. The flu season and travel restrictions have impacted the market, with tourism and travel industries taking a hit. Mask mandates and social distancing measures have increased the need for COVID Testing Kits. Multiplex NAATs are gaining popularity for tracing and testing infected individuals, including recovered patients. Technological advancements have led to the development of rapid testing kits and immunoassay test strips. The market is witnessing regulatory approvals from governments and private funds for emergency use authorizations of non-medical devices as COVID-19 cases continue to rise. Hospitals and clinics, along with diagnostic laboratories, are at the forefront of this battle against infectious diseases, including respiratory illnesses like COVID-19, flu, and respiratory syncytial virus. The market for COVID-19 testing kits is expected to grow significantly due to the ongoing pandemic, with hospitals and clinics, and diagnostic laboratories playing a crucial role in providing testing services. The market for COVID-19 testing kits is expected to grow significantly due to the ongoing pandemic, with hospitals and clinics, and diagnostic laboratories playing a crucial role in providing testing services. The government of Singapore has also approved the use of antigen rapid tests for COVID-19 detection. The market for COVID-19 testing kits is expected to continue growing as the world battles various infectious pathogens.
Request Sample
of our comprehensive report now to stay ahead in the AI-driven market evolution!
Market
Challenges
-
The coronavirus test kits market in North America is experiencing challenges due to the circulation of counterfeit products. Widespread testing is crucial for controlling the COVID-19 pandemic, but the unauthorized sale of fake test kits poses risks to consumer health and the reputation of established market players. US Customs and Border Protection has seized several shipments of suspected counterfeit test kits arriving from the UK. Health Canada also warned against the use of unlicensed COVID-19 test kits from Health Advance Inc. Sold through Healthful Plus. The complex supply chain and fragmented wholesale market facilitate the sale of counterfeit test kits, which can lead to financial losses, brand damage, and potential health risks. These substandard kits may not comply with FDA and
EU standards, causing bacterial contamination and decreased product reliability. The prevalence of counterfeit test kits may deter end-users from adopting coronavirus testing and hinder the growth of the coronavirus test kits market in North America. The Coronavirus Test Kits market in North America faces several challenges in the current scenario. Obtaining private funds for mass production and distribution of these kits is a significant hurdle. Emergency Use Authorizations (EUAs) for non-medical devices are necessary, but discrepancies in testing protocols for infectious diseases like COVID-19, flu, RSV, and other respiratory illnesses can lead to inaccurate results. Healthcare workers (HCWs) require reliable testing to ensure patient safety and trace and test recovered patients. Immunoassay test strips are essential for rapid testing, but sensitivity and accuracy are crucial factors. Technological advancements, such as nasopharyngeal, oropharyngeal, and nasal swabs, are improving the testing process. Hospitals, clinics, diagnostic laboratories, and government agencies require various testing solutions for infectious diseases, genetic disorders, and pathogen detection in fields like oncology, forensic sciences, and food microbiology. The mortality rate focuses the need for reliable and accurate testing. Rapid testing kits from the Government of Singapore's antigen rapid test and other major infectious disease testing companies are essential to combat the spread of infectious pathogens. Laboratory techniques, such as DNA-based methods, are essential for accurate results. Overall, the Coronavirus Test Kits market in North America requires a response from healthcare providers and product portfolios to meet the demands of various industries and sectors.
Discover how AI is revolutionizing market trends-
Get your access now!
Segment Overview
This coronavirus test kits market in North America report extensively covers market segmentation by
-
1.1 Government
1.2 Non-government
-
2.1 Rapid test kit
2.2 RT-PCR
2.3 Others
-
3.1 North America
1.1
Government-
The Coronavirus Test Kits market in North America has experienced significant growth due to the massive demand from various levels of government for COVID-19 testing. In response, governments have implemented testing programs and initiatives to monitor and control the spread of the virus. For instance, the White House introduced COVIDTests in January 2022, allowing all American households to order free at-home test kits. The CDC also distributed over 200 test kits in February 2020, which could test around 150,000 patients. These initiatives require a substantial number of test kits, driving market growth. The CDC's CDC 2019-nCoV RT-PCR test kit, used for upper and lower respiratory specimens, is not FDA-approved but is distributed through the International Reagent Resource (IRR) to qualified laboratories, including 15 in the US and 191 internationally. The high demand from governments for coronavirus test kits has fueled market growth, but this demand is expected to decrease due to successful vaccination programs.
Download a Sample
of our comprehensive report today to discover how AI-driven innovations are reshaping competitive dynamics
Research Analysis
The Coronavirus market in North America has seen a significant growth due to the ongoing COVID-19 pandemic caused by the SARS CoV 2 virus. RT PCR tests have been the primary diagnostic technique for COVID-19, with COVID-19 Test Kits in high demand. However, the need for rapid testing kits has also increased to address the backlog and discrepancies in testing results. The market for COVID-19 testing kits is not limited to medical devices but also includes non-medical devices such as antigen rapid tests. The market is driven by the increasing number of COVID-19 cases, government initiatives, private funds, and emergency use authorizations. The market for COVID-19 testing kits is not just limited to infectious diseases but also extends to genetic disorders. Healthcare providers are expanding their product portfolios to cater to the growing demand. The mortality rate of COVID-19 and the need for booster doses have further fueled the demand for testing kits. The government of Singapore is also a significant player in the market, having developed its own rapid test for COVID-19.
Market Research Overview
The Coronavirus market in North America is currently focused on the production and distribution of RT PCR tests for COVID-19. Diagnostic techniques for COVID-19 have been a priority during the ongoing pandemic, with immunization coverage and booster doses also playing a crucial role in managing the situation. The flu season and travel restrictions have impacted the demand for COVID Testing Kits, with mask mandates and social distancing measures becoming the new norm. Multiplex NAATs have emerged as a technological advancement in infectious disease testing, allowing for the detection of multiple pathogens at once. The market also includes COVID 19 cases, government and private funds, emergency use authorizations for non-medical devices, and healthcare providers. Discrepancies in testing protocols and tracing and testing of recovered patients are areas of ongoing concern. Immunoassay test strips, flu, respiratory syncytial virus, and other respiratory illnesses are also tested for in hospitals and clinics, diagnostic laboratories, and other healthcare facilities. The market includes various product portfolios, with sensitivity and accuracy being key factors in the selection of testing kits. The mortality rate of major infectious diseases, rapid testing kits, antigen rapid tests, and infectious pathogen detection are also important aspects of the market. DNA-based laboratory techniques, human genetic testing, forensic sciences, oncology testing, blood screening, and food microbiology are other areas where the market intersects. The government of Singapore and other countries have played a significant role in funding and regulating the market.
Table of Contents:
1 Executive Summary
2 Market Landscape
3 Market Sizing
4 Historic Market Size
5 Five Forces Analysis
6 Market Segmentation
-
End-user
-
Government
Non-government
-
Rapid Test Kit
RT-PCR
Others
-
North America
7 Customer Landscape
8 Geographic Landscape
9 Drivers, Challenges, and Trends
10 Company Landscape
11 Company Analysis
12 Appendix
About Technavio
Technavio is a leading global technology research and advisory company. Their research and analysis focuses on emerging market trends and provides actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions.
With over 500 specialized analysts, Technavio's report library consists of more than 17,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio's comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios.
Contacts
Technavio Research
Jesse Maida
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
Email:
[email protected]
Website:
SOURCE Technavio
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE? 440k+Newsrooms &
Influencers 9k+
Digital Media
Outlets 270k+
Journalists
Opted In GET STARTED

Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.
















Comments
No comment