(MENAFN- EIN Presswire) North America IVD Regulatory Affairs Outsourcing Market
North America IVD regulatory affairs outsourcing market is expected to gain market growth in the forecast period of 2022 to 2029.
PUNE, MAHARASHTRA, INDIA, December 22, 2022 /einpresswire.com / -- north america ivd regulatory affairs outsourcing market report discusses market trends and analyses the impact of buyers, substitutes, new entrants, competitors, and suppliers on the North America IVD Regulatory Affairs Outsourcing industry. The market report makes available data on patterns and improvements, target business sectors and materials, limits and advancements.
This market research document contains data and information about the scenario of North America IVD Regulatory Affairs Outsourcing industry which makes it easy to move ahead of the competition in today's rapidly changing business environment. Estimation of strategic options, suggestions of winning action plans and support to make critical bottom-line decisions is also given in North America IVD Regulatory Affairs Outsourcing market report by experienced and innovative industry experts.
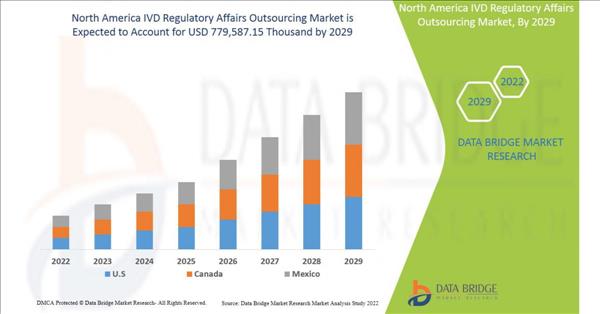
North America IVD regulatory affairs outsourcing market is expected to gain market growth in the forecast period of 2022 to 2029. Data Bridge Market Research analyses that the market is growing with the CAGR of 13.4% in the forecast period of 2022 to 2029 and expected to reach USD 779,587.15 thousand by 2029.
Grab Sample Report with Complete Graphs, Charts and Figures @
Market Outline: -
In vitro diagnostic products are reagents, devices, and systems used to diagnose disease or other conditions, including determining one's state of health to cure, mitigate, treat, or prevent disease. These products are intended for use in the collecting, preparation, and examination of human body specimens. Regulatory affairs play a crucial part in the in vitro diagnostic device (IVD) and medical device industry.
The regulatory affairs outsourcing services entails medical writing and publication of regulatory documentation by professionals who contribute to the production of high-quality documents for clinical research projects. The demand for regulatory services outsourcing is substantially increasing in clinical studies conducted in emerging economies, providing a healthy platform for this industry's growth.
The major factors driving the growth of the IVD regulatory affairs outsourcing market are development of project based support leads to long term outsourcing agreement among organization and technological advancement in various in vitro diagnostic devices. Increase in R&D activities by companies across the region is creating opportunities for the growth of the market. Higher cost related to maintenance and outsourcing of IVD is acting as the major restraint for IVD regulatory affairs outsourcing market. Shortage of skilled personnel for handling in vitro diagnostic devices is acting as a major challenge for the growth of the market.
Some of the major players operating in the report are IVD regulatory affairs outsourcing market are Freyr Solutions, AXSource, LORENZ Life Sciences Group, PPD Inc. (A Subsidiary of Thremofisher Scientific Inc.), Promedica International, a California Corporation, Assent Compliance Inc., MakroCare, EMERGO, ICON, Parexel International Corporation, CRITERIUM, INC., Groupe ProductLife S.A., Propharma Group, VCLS, Labcorp Drug Development, WuXi AppTec, Charles River Laboratories, Genpact, Medpace, Regulatory Compliance Associates Inc., RQM+, Saraca Solutions Private Limited, PBC BioMed, Dor Pharmaceutical Services, Qserve, mdiConsultants, Inc. among others. DBMR analysts understand competitive strengths and provide competitive analysis for each competitor separately.
Access Full 350 Pages PDF Report @
north america ivd regulatory affairs outsourcing market scope and Market Size
North America IVD regulatory affairs outsourcing market is segmented into seven notable segments which are based on the services, indication, deployment mode, organization size, stage, class and end user.
On the basis of services, the North America IVD regulatory affairs outsourcing market is segmented into regulatory writing & submissions, regulatory registration & clinical trial applications, regulatory consulting, legal representation, data management services, chemistry manufacturing and controls (CMC) services, and others. In 2022, regulatory writing & submissions is expected to dominate the market as the industry is responding to several widespread developments, which have triggered innovations and expansion in IVD technologies. These large-scale trends include an aging population, an increase in the occurrence of infectious diseases, the influence of big tech innovators and acceptance of personalized care, and desire for ease of use.
On the basis of indication, the North America IVD regulatory affairs outsourcing market is segmented into oncology, neurology, cardiology, clinical chemistry and immunoassays, precision medicine, infectious diseases, diabetes, genetic testing, HIV/AIDS, haematology, drug testing/pharmacogenomics, blood transfusion, point of care, and others. In 2022, the oncology segment is expected to dominate as it improves the predictability of the oncology drug development process and becomes a useful tool for oncologists when deciding on a treatment plan for a patient
On the basis of deployment mode, the North America IVD regulatory affairs outsourcing market is segmented into cloud and on-premises. In 2022, the cloud segment is expected to dominate as it Cloud computing technology in IVD regulatory gives a stable infrastructure with maximum output to IVD systems dealing firms due to its cost-effective and solution-flexible properties.
On the basis of organization size, the North America IVD regulatory affairs outsourcing market is segmented into small and medium enterprises (SMES) and large enterprises. In 2022, the large enterprises segment is expected to dominate as it involvement of the peer high quality technology and service portfolio related to the IVD regulatory.
On the basis of stage, the North America IVD regulatory affairs outsourcing market is segmented into clinical, preclinical, and PMA (post-market authorization). In 2022, clinical segment is expected to dominate the market because clinical trials require clearance from authorities, every result must be filed and thoroughly recorded before submissions, regulatory affairs services are in high demand in this area.
On the basis of class, the North America IVD regulatory affairs outsourcing market is segmented into class I, class II, and class III. In 2022, class I segment is expected to dominate the market as it involves no public health risk or low personal risk with lowest regulations. These are high in demand and 47% of medical devices fall under this category.
On the basis of end user, the North America IVD regulatory affairs outsourcing market is segmented into pharmaceutical companies, medical device companies, biotechnology companies, and others. In 2022, medical device companies are expected to dominate the market by emerging in various efficient technological services and standards.
Table of Content:
Section 01: Executive Summary
Section 02: Scope of The Report
Section 03: Research Methodology
Section 04: Introduction
Section 05: Market Landscape
Section 06: Market Sizing
Section 07: Five Forces Analysis
Section 08: Market Segmentation by Product
Section 09: Market Segmentation by Application
Section 10: Customer Landscape
Section 11: Market Segmentation by End-User
Section 12: Regional Landscape
Section 13: Decision Framework
Section 14: Drivers and Challenges
Section 15: Market Trends
Section 16: Competitive Landscape
Section 17: Company Profiles
Section 18: Appendix
For More Insights Get Detailed TOC @
Frequently asked questions:
What is the Forecast Market Value for North America IVD Regulatory Affairs Outsourcing Market?
What is the Growth Rate of the North America IVD Regulatory Affairs Outsourcing Market?
Which are the Major Market Drivers for North America IVD Regulatory Affairs Outsourcing Market?
What are the Major Companies Operating in the North America IVD Regulatory Affairs Outsourcing Market?
Which Countries Data is covered in the North America IVD Regulatory Affairs Outsourcing Market?
Browse Trending Reports:
North America Whole Exome Sequencing Market – Industry Trends and Forecast to 2029
North America Health Tourism Market – Industry Trends and Forecast to 2029
North America Proton Therapy Market – Industry Trends and Forecast to 2028
North America Psychedelic Drugs Market – Industry Trends and Forecast to 2029
North America Gene Synthesis Market – Industry Trends and Forecast to 2029
North America Dry Eye Syndrome Market – Industry Trends and Forecast to 2029
About Data Bridge Market Research:
Data Bridge set forth itself as an unconventional and neoteric Market research and consulting firm with unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process.Sopan Gedam
Data Bridge Market Research
+1 888-387-2818
email us here
MENAFN21122022003118003196ID1105339169
Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.



















Comments
No comment