
403
Sorry!!
Error! We're sorry, but the page you were looking for doesn't exist.
Electroducer announces the publication of positive results from its pilot study in EuroIntervention medical journal
(MENAFN- Andrew Lloyd & Associates) - The first of its kind study involved 60 patients and confirmed Electroducer Sleeve® as a safe and effective treatment for heart diseases
- The company plans to launch its device in the US market by the end of 2023, followed by the European market in 2024
Grenoble, France, December 15, 2022 - Electroducer, a company developing an innovative and cost-effective medical device for the treatment of heart diseases, today announces that the positive results from its pilot study have been published in the medical journal EuroIntervention.
EuroIntervention is well regarded globally as the most prestigious European publication focused on interventional cardiology.
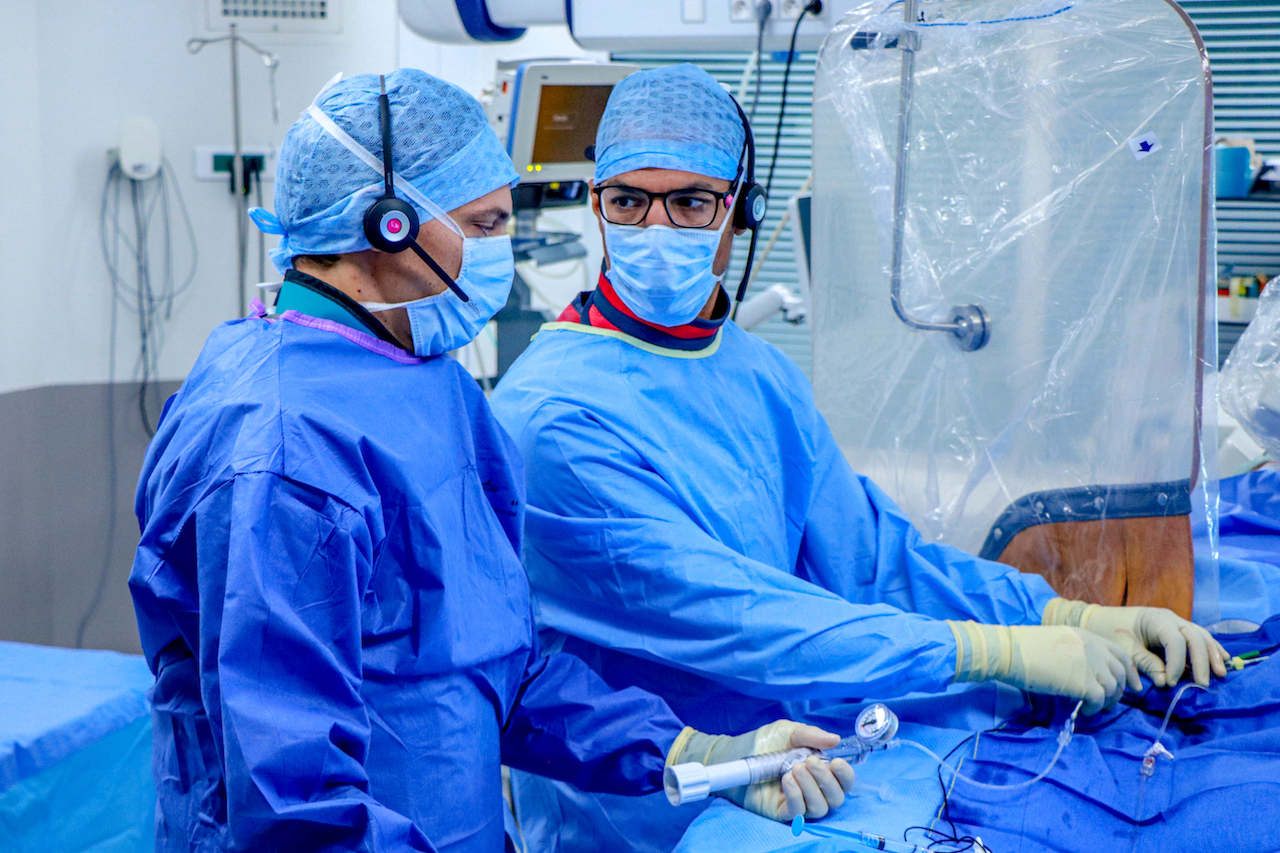
Entitled ‘A direct wire pacing device for transcatheter heart valve and coronary interventions: a first-in-human, multicentre study of the Electroducer Sleeve’, the study involved 60 patients from four French medical centers (the Clinique Pasteur in Toulouse, the Cardiovascular Institute in Grenoble, the Médipôle Lyon-Villeurbanne Hospital in Villeurbanne and the Jacques Cartier private Hospital in Massy). It was the first of its kind in the world and confirmed that the Electroducer Sleeve® is a safe and effective treatment for heart valve diseases and complex coronary interventions.
The Electroducer Sleeve® makes it simpler and safer to perform endovascular procedures requiring temporary cardiac pacing. Implanting a temporary pacemaker is the current conventional technique. This is an invasive procedure that carries additional risks and increases procedural time and radiation exposure for the patient. In the future, the Electroducer Sleeve® could replace this step by enabling the heart to be stimulated directly through the ‘guidewire’, which is used to deliver the valve or stent. This combined approach simplifies the procedure and reduces the risk of complications at the same time. According to a study published in 20191, it also reduces the total cost of the procedure by around 12%.
Dr. Jérôme Wintzer-Wekehind, principal investigator on the study and interventional cardiologist at the Cardiovascular Institute in Grenoble, said: “As well as offering benefits for patients, this device is simple and universal — these advantages mean there will certainly be widespread uptake and usage.”
Dr. Nicolas Dumonteil, one of the investigators on the study and interventional cardiologist at the Clinique Pasteur in Toulouse, added: “I am confident that in a few years’ time, Electroducer Sleeve® will replace our conventional technique, providing physicians and patients with a faster, safer and simpler procedure.”
Dr. Benjamin Faurie, CEO and founder of Electroducer, said: “Having our study results published in such a prestigious medical journal shows that our clinical approach has tremendous potential to become the procedure of choice. It is a great opportunity to present the benefits of our solution to the global cardiology community. We plan to bring the Electroducer Sleeve® to market within the next year, so that as many patients and cardiologists as possible can take advantage of our technology.”
The Electroducer Sleeve® is expected to reach the US market in 2023, followed by the European market in 2024, once it receives FDA authorization and a CE marking respectively. The device has the potential to be used in nearly 1.5 million interventions per year worldwide by 2025. The company aims to capture 8% of this market, representing around 120,000 procedures per year, by this date.
About Electroducer
Founded in 2018 by Dr. Benjamin Faurie, Electroducer designs and develops a world-first medical device, Electroducer Sleeve®, specifically to treat heart malfunctions (myocardial infarction, heart valve disease, etc.) requiring a valve replacement, or percutaneous coronary interventions (treatment of the arteries that supply the heart).
The device is based on a medical technique known as Direct Wire Pacing (DWP®), which was also developed by the company’s founder and used for the first time in 2011. The technique uses the ‘guidewire’ — the instrument used to deliver the replacement valve — to directly stimulate the heart muscle, eliminating the need for a temporary pacemaker and all the associated complications, which in 2–6% of cases can be serious. It has proven to be simpler, faster and cheaper than the conventional technique, with less radiation, and as effective.
Direct Wire Pacing (DWP®) is a pioneering technique for Transcatheter Aortic Valve Implantation (TAVI) with proven advantages over the conventional approach, as confirmed by the randomized multi-center EASY TAVI study (JACC 20191). In the case of TAVI, DWP helps limit per- and post-operative trauma by significantly reducing the duration and the invasive nature of the procedure. For physicians, the technique simplifies the procedure and makes it safer, without any need to change their existing practices. For hospitals, meanwhile, it represents a real cost saving of around 12% compared to the conventional approach.
However, Direct Wire Pacing has some technical limitations that hinder widespread adoption. The goal of the Electroducer Sleeve® is therefore to increase the number of patients with access to this revolutionary technique by providing a universal, easy-to-use, turnkey device.
The Electroducer Sleeve®, which is covered by 25 international patents, will be marketed in the US and Europe by the end of 2023 and in 2024 respectively. Initially, the device will be used for TAVI and complex coronary procedures (700,000 per year worldwide). By 2025, the technology will also be used in mitral and tricuspid valves replacement, numbering nearly 1.5 million procedures per year.
Research and development work is also underway, looking into some other highly promising applications around neuromodulation and the non-pharmacological treatment of hypertension.
Electroducer is based in Grenoble, France, and is supported financially by public sector financing bodies (French public investment bank Bpifrance, the Auvergne-Rhône-Alpes regional authority and the French Ministry of Higher Education, Research and Innovation) and private investors.
1 Benjamin Faurie, Géraud Souteyrand, Patrick Staat, Matthieu Godin, Christophe Caussin, Eric Van Belle, Lionel Mangin, Pierre Meyer, Nicolas Dumonteil, Mohamed Abdellaoui, Jacques Monségu, Isabelle Durand-Zaleski, Thierry Lefèvre and for the EASY TAVI Investigators. Left Ventricular Rapid Pacing Via the Valve Delivery Guidewire in Transcatheter Aortic Valve Replacement, Journal of American College of Cardiology (JACC), Volume 12, Issue 24 (2449-2459), December 2019.
- The company plans to launch its device in the US market by the end of 2023, followed by the European market in 2024
Grenoble, France, December 15, 2022 - Electroducer, a company developing an innovative and cost-effective medical device for the treatment of heart diseases, today announces that the positive results from its pilot study have been published in the medical journal EuroIntervention.
EuroIntervention is well regarded globally as the most prestigious European publication focused on interventional cardiology.
Entitled ‘A direct wire pacing device for transcatheter heart valve and coronary interventions: a first-in-human, multicentre study of the Electroducer Sleeve’, the study involved 60 patients from four French medical centers (the Clinique Pasteur in Toulouse, the Cardiovascular Institute in Grenoble, the Médipôle Lyon-Villeurbanne Hospital in Villeurbanne and the Jacques Cartier private Hospital in Massy). It was the first of its kind in the world and confirmed that the Electroducer Sleeve® is a safe and effective treatment for heart valve diseases and complex coronary interventions.
The Electroducer Sleeve® makes it simpler and safer to perform endovascular procedures requiring temporary cardiac pacing. Implanting a temporary pacemaker is the current conventional technique. This is an invasive procedure that carries additional risks and increases procedural time and radiation exposure for the patient. In the future, the Electroducer Sleeve® could replace this step by enabling the heart to be stimulated directly through the ‘guidewire’, which is used to deliver the valve or stent. This combined approach simplifies the procedure and reduces the risk of complications at the same time. According to a study published in 20191, it also reduces the total cost of the procedure by around 12%.
Dr. Jérôme Wintzer-Wekehind, principal investigator on the study and interventional cardiologist at the Cardiovascular Institute in Grenoble, said: “As well as offering benefits for patients, this device is simple and universal — these advantages mean there will certainly be widespread uptake and usage.”
Dr. Nicolas Dumonteil, one of the investigators on the study and interventional cardiologist at the Clinique Pasteur in Toulouse, added: “I am confident that in a few years’ time, Electroducer Sleeve® will replace our conventional technique, providing physicians and patients with a faster, safer and simpler procedure.”
Dr. Benjamin Faurie, CEO and founder of Electroducer, said: “Having our study results published in such a prestigious medical journal shows that our clinical approach has tremendous potential to become the procedure of choice. It is a great opportunity to present the benefits of our solution to the global cardiology community. We plan to bring the Electroducer Sleeve® to market within the next year, so that as many patients and cardiologists as possible can take advantage of our technology.”
The Electroducer Sleeve® is expected to reach the US market in 2023, followed by the European market in 2024, once it receives FDA authorization and a CE marking respectively. The device has the potential to be used in nearly 1.5 million interventions per year worldwide by 2025. The company aims to capture 8% of this market, representing around 120,000 procedures per year, by this date.
About Electroducer
Founded in 2018 by Dr. Benjamin Faurie, Electroducer designs and develops a world-first medical device, Electroducer Sleeve®, specifically to treat heart malfunctions (myocardial infarction, heart valve disease, etc.) requiring a valve replacement, or percutaneous coronary interventions (treatment of the arteries that supply the heart).
The device is based on a medical technique known as Direct Wire Pacing (DWP®), which was also developed by the company’s founder and used for the first time in 2011. The technique uses the ‘guidewire’ — the instrument used to deliver the replacement valve — to directly stimulate the heart muscle, eliminating the need for a temporary pacemaker and all the associated complications, which in 2–6% of cases can be serious. It has proven to be simpler, faster and cheaper than the conventional technique, with less radiation, and as effective.
Direct Wire Pacing (DWP®) is a pioneering technique for Transcatheter Aortic Valve Implantation (TAVI) with proven advantages over the conventional approach, as confirmed by the randomized multi-center EASY TAVI study (JACC 20191). In the case of TAVI, DWP helps limit per- and post-operative trauma by significantly reducing the duration and the invasive nature of the procedure. For physicians, the technique simplifies the procedure and makes it safer, without any need to change their existing practices. For hospitals, meanwhile, it represents a real cost saving of around 12% compared to the conventional approach.
However, Direct Wire Pacing has some technical limitations that hinder widespread adoption. The goal of the Electroducer Sleeve® is therefore to increase the number of patients with access to this revolutionary technique by providing a universal, easy-to-use, turnkey device.
The Electroducer Sleeve®, which is covered by 25 international patents, will be marketed in the US and Europe by the end of 2023 and in 2024 respectively. Initially, the device will be used for TAVI and complex coronary procedures (700,000 per year worldwide). By 2025, the technology will also be used in mitral and tricuspid valves replacement, numbering nearly 1.5 million procedures per year.
Research and development work is also underway, looking into some other highly promising applications around neuromodulation and the non-pharmacological treatment of hypertension.
Electroducer is based in Grenoble, France, and is supported financially by public sector financing bodies (French public investment bank Bpifrance, the Auvergne-Rhône-Alpes regional authority and the French Ministry of Higher Education, Research and Innovation) and private investors.
1 Benjamin Faurie, Géraud Souteyrand, Patrick Staat, Matthieu Godin, Christophe Caussin, Eric Van Belle, Lionel Mangin, Pierre Meyer, Nicolas Dumonteil, Mohamed Abdellaoui, Jacques Monségu, Isabelle Durand-Zaleski, Thierry Lefèvre and for the EASY TAVI Investigators. Left Ventricular Rapid Pacing Via the Valve Delivery Guidewire in Transcatheter Aortic Valve Replacement, Journal of American College of Cardiology (JACC), Volume 12, Issue 24 (2449-2459), December 2019.
Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.

















Comments
No comment