(
MENAFN- PR Newswire)
IRVINE, Calif., Dec. 19, 2024 /PRNewswire/ -- OrthAlign, Inc. today announced a significant milestone with the successful first clinical use of its Lantern Hip handheld technology. The procedure was performed by Edwin
Su, MD, a renowned orthopedic surgeon at the Hospital for Special Surgery (HSS) in New York, NY. "The first clinical case of Lantern Hip is a monumental achievement for our team and the surgeons involved with this project," said Eric Timko, CEO of OrthAlign. "This expansion of our flagship platform to include hips not only enhances our product portfolio, but also positions us for significant growth in both the hospital and the ambulatory surgery center (ASC). We're excited to kick off the new year with Lantern Hip and showcase its impact at the American Academy of Orthopaedic Surgeons annual meeting," says Eric Timko.
Continue Reading

Edwin Su, MD, Orthopedic Surgeon at Hospital for Special Surgery performed the first Lantern Hip procedure.
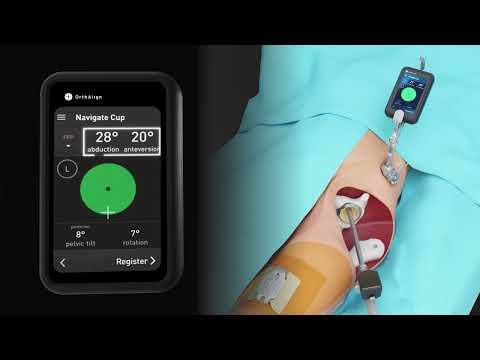
Meet Lantern Hip. The next evolution in hip technology is in your hands.
Lantern Hip is the latest evolution in hip technology, built upon the success of over 375,000 OrthAlign procedures worldwide. Next-generation sensors, powered by accelerometers and gyroscopes, are designed to provide an accurate and simple solution to navigate cup placement and measure changes in leg length and offset. The system enables the surgeon to choose their preferred implant, and is accessible to any site of service.
"Lantern Hip allows me to personalize cup position for each patient," said Dr. Su. "I can compare the functional pelvic plane (FPP), the anterior pelvic plane (APP), and the coronal plane during live cup navigation, so I can place the implant in the best position for function and stability. With its triple-sensor technology, Lantern Hip also allows me to feel confident in my leg length and offset restoration. The system was simple for me and my team to integrate into our workflow during our first case, and I expect this will make a positive impact on other surgeons' experience too."
OrthAlign will continue to offer surgeons the opportunity to experience Lantern Hip firsthand through webinars and demonstrations at industry events throughout 2025. For inquiries about upcoming events or to schedule a product demonstration, contact your local OrthAlign representative.
Visit
to view the Lantern Hip introductory video.
Lantern Hip is indicated for use in direct anterior total hip arthroplasty procedures with the patient in the supine position.
About OrthAlign, Inc.
OrthAlign is a medical device company with a focus on delivering practical, cutting-edge technologies for orthopedic surgery. With a commitment to innovation and excellence, OrthAlign provides surgeons with user-friendly, cost-effective solutions to help improve patient care in joint replacement. In 2023, the company celebrated a record-breaking year with over $50 million in global revenue, reflecting its dedication to growth and leadership in the industry.
Driven by the belief that everyone deserves exceptional healthcare, OrthAlign is committed to making empowering technologies accessible to all.
LANTERN® and ORTHALIGN® are registered trademarks of OrthAlign, Inc.
SOURCE OrthAlign
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE?
440k+
Newsrooms &
Influencers
9k+
Digital Media
Outlets
270k+
Journalists
Opted In
GET STARTED
MENAFN19122024003732001241ID1109014068
Legal Disclaimer:
MENAFN provides the information “as is” without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the provider above.