(MENAFN- PR Newswire)
BERKELEY, Calif., Oct. 23, 2024 /PRNewswire/ --
ResVita Bio, a synthetic biology company developing innovative therapies for skin diseases, today announced a productive FDA INTERACT meeting held on August 10, 2024. The meeting focused on the development plan for RVB-003, ResVita Bio's investigational therapy for Netherton Syndrome, a chronic and life-threatening genetic skin disorder. This milestone underscores the alignment between the FDA and ResVita Bio's new platform for continuous protein therapy-a groundbreaking therapeutic approach designed to deliver sustained drug levels directly to the skin, offering potentially unparalleled safety and efficacy for treatment of skin diseases.
Continue Reading
ResVita Bio
Netherton Syndrome is a rare genetic disorder characterized by chronic inflammation, intense itching, and atopic manifestations. Compromised skin integrity often leads to infections and dehydration, contributing to high post-natal mortality rates. This condition is caused by mutations in the SPINK5 gene, which leads to uncontrolled protease activity and premature exfoliation of the outer skin layers.
During the meeting, the FDA provided valuable feedback on key aspects of the RVB-003 program, including biocontainment; chemistry, manufacturing, and controls; the preclinical program; and preclinical safety studies. ResVita Bio plans to incorporate this feedback as it moves towards submitting a Pre-IND (Investigational New Drug) application for RVB-003 in 2025.
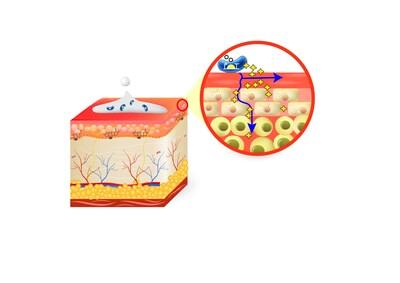
RVB-003 is based on ResVita Bio's continuous protein therapy platform, which utilizes genetically engineered bacteria applied topically. This novel approach aims to address the short lifespan of therapeutic proteins by continuously producing them directly on the skin surface, thereby enhancing efficacy and safety profiles. ResVita Bio presented the completion of in vitro, ex vivo, and in vivo efficacy proof-of-concept studies during the meeting, demonstrating RVB-003's potential to treat Netherton Syndrome.
"We are very encouraged by the outcome of our INTERACT meeting with the FDA," said Amin Zargar, CEO of ResVita Bio. "The strong alignment between our development approach and the feedback from FDA reviewers reinforces our confidence in RVB-003's expedited path to the clinic. We look forward to incorporating their guidance as we advance towards our IND submission."
About ResVita Bio
ResVita Bio is a biotech startup based in Berkeley, California, dedicated to revolutionizing the treatment of skin diseases through its continuous protein therapy platform. By utilizing genetically engineered bacteria to deliver continuous therapeutic proteins topically, ResVita Bio aims to enhance the efficacy and safety profiles of its therapies, offering new hope for patients worldwide. This development is supported by private investment and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) awards 1R43AR082240-01 and 2R44AR082240-02.
Media Contact:
Amin Zargar
510-328-6703
[email protected]
SOURCE ResVita Bio
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE?
440k+
Newsrooms &
Influencers
9k+
Digital Media
Outlets
270k+
Journalists
Opted In
GET STARTED
MENAFN23102024003732001241ID1108811043
Legal Disclaimer:
MENAFN provides the information “as is” without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the provider above.