(MENAFN- PR Newswire)
New 'plus' version of test adds a 3rd gene target to enhance detection of future SARS-CoV-2 variants
SUNNYVALE, Calif., May 12, 2022 /PRNewswire/ -- Cepheid today announced it has received Emergency Use Authorization (EUA) from the U.S. Food & Drug Administration (FDA) for Xpert® Xpress CoV-2 plus , a rapid molecular diagnostic test for qualitative detection of the virus that causes COVID-19.
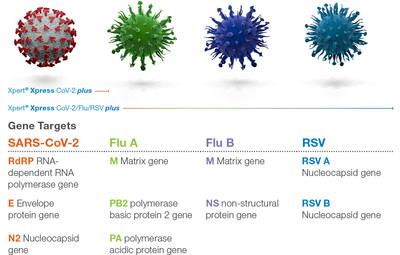
Gene Targets

Xpert® Xpress CoV-2 plus Cartridge
>1. PPA and NPA for asymptomatic specimens were calculated using anterior nasal swab specimens
2. With early assay termination for positives only; reporting of negatives in approximately 30 minutes
About Cepheid Based in Sunnyvale, Calif., Cepheid is a leading molecular diagnostics company. Cepheid is dedicated to improving healthcare by developing, manufacturing, and marketing accurate yet easy-to-use molecular systems and tests. By automating highly complex and time-consuming manual procedures, the company's solutions deliver a better way for institutions of any size to perform sophisticated molecular diagnostic testing for organisms and genetic-based diseases. Through its strong molecular biology capabilities, the company is focusing on those applications where accurate, rapid, and actionable test results are needed most, such as managing infectious diseases and cancer. For more information, visit .
About Emergency Use Authorization Status This product has not been FDA cleared or approved, but has been authorized by FDA under an EUA for use by authorized laboratories. This product has been authorized only for the detection of nucleic acid from SARS-CoV-2, not for any other viruses or pathogens. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
For Cepheid Media Inquiries: Darwa Peterson [email protected]
SOURCE Cepheid
MENAFN12052022003732001241ID1104201629
Legal Disclaimer:
MENAFN provides the information “as is” without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the provider above.