
Real-World Data (RWD) Market Size Worth USD 6.37 Bn By 2034 Driven By AI Adoption, Precision Medicine, And Drug Development Demand
| Report Coverage | Details | |
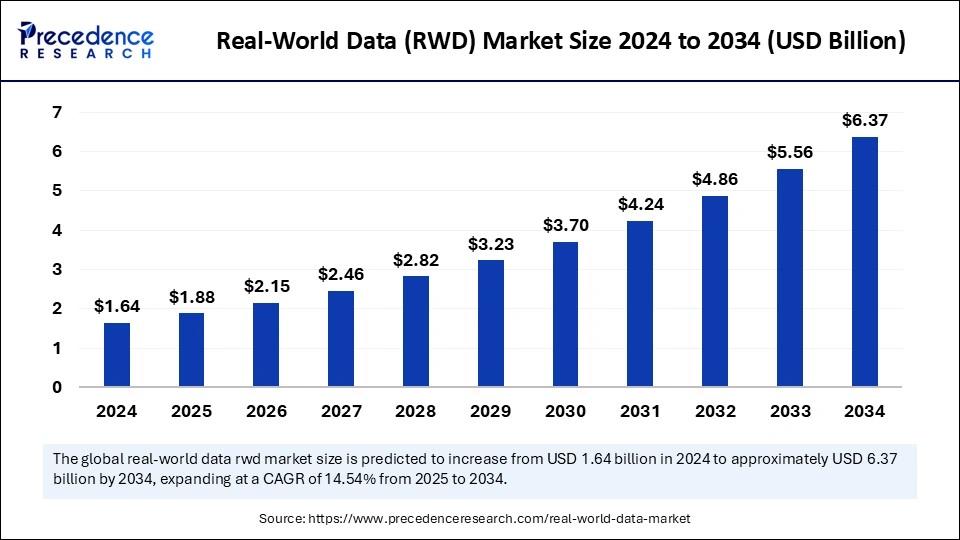
| Market Size (2025) | USD 1.88 Billion | |
| Market Size (2026) | USD 2.15 Billion | |
| Market Size (2034) | USD 6.37 Billion | |
| CAGR (2025–2034) | 14.54% | |
| Dominating Region | North America | |
| Fastest Growing Region | Asia Pacific | |
| Base Year | 2024 | |
| Forecast Period | 2025 to 2034 | |
| Segments Covered | Component, Application, End User, and Regions | |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, Middle East & Africa | |
| Key Components | Services (largest share), Datasets (fastest growing) | |
| Top Applications | Drug Development & Approvals; Post-Market Surveillance | |
| Major End Users | Pharmaceutical & Medical Device Companies; Healthcare Providers | |
| Key Market Drivers | Rise of evidence-based medicine, telehealth expansion, decentralized trials, growth in precision medicine, increased wearable/IoT health data, regulatory support for RWE | |
| Challenges | Data interoperability, lack of standardization across sources | |
| Opportunities | Increasing RWE use in approvals, demand for post-market safety data, value-based care adoption, chronic disease management insights | |
| Notable Market Players | IQVIA, Optum, Flatiron Health, IBM, SAS, Palantir, Tempus, Evidera |
Immediate Delivery Available | Buy This Premium Research Report@
Real-World Data (RWD) Market Regional Outlook
Why North America Dominates the Real-World Data (RWD) Market?
North America dominated the market with a 43% share in 2024. The strong presence of advanced healthcare infrastructure and high adoption of EHRs increases demand for RWD. The high prevalence of chronic diseases and a well-established pharmaceutical sector increase the adoption of RWD. The supportive government policies, like the U.S. FDA and a shift towards value-based care, drive the overall market growth.
What is the U.S. Real-World Data (RWD) Market Size and Growth?
According to Precedence Research, the U.S. real-world data (rwd) market size is valued at USD 610 million in 2025 and is expected to surpass USD 2,090 million by 2034, with a noteworthy CAGR of 14.70% from 2025 to 2034.
U.S. Real-World Data (RWD) Market Trends
The U.S. market is expanding as healthcare systems, insurers, and biopharma companies increasingly use real-world evidence to optimize clinical outcomes and reduce costs. Growing integration of electronic health records, wearable devices, and claims data is enhancing the depth and accuracy of patient insights.
The shift towards value-based care is accelerating demand for advanced RWD analytics to support treatment effectiveness assessments and reimbursement decisions.
Note: This report is readily available for immediate delivery. We can review it with you in a meeting to ensure data reliability and quality for decision-making.Try Before You Buy – Get the Sample Report@
How is the Asia Pacific experiencing the Fastest Growth in the Real-World Data (RWD) Market?
Asia Pacific is experiencing the fastest growth in the market during the forecast period. The growing prevalence of chronic diseases and increasing life expectancy increase demand for RWD. The strong government support for RWD use in countries like China & Japan, and increasing investment in analytics solutions & digital health, help market growth. The strong presence of clinical trial landscapes and technological innovations like cloud-based analytics, the rise of big data, & artificial intelligence
China Real-World Data (RWD) Market Trends
China's market is growing rapidly as healthcare providers and pharmaceutical companies increasingly rely on real-world evidence to support clinical decision-making and accelerate drug development. Expanded digital health infrastructure, including widespread electronic medical records and government-backed health data platforms, is enabling more efficient data collection and integration.
Real-World Data (RWD) Market Segmentation Insights
Component Insights
Why the Services Segment Held the Largest Share in the Real-World Data (RWD) Market?
The services segment held the largest revenue share of the market in 2024. The well-established pharmaceutical sector and growing development of drugs increase demand for services. The growing shift towards value-based care and a strong focus on product approvals increases the adoption of services. The rise in clinical trials
The datasets segment is the fastest-growing in the market during the forecast period. The growing adoption of wearable devices and the adoption of electronic health records increases the production of datasets. The strong focus on drug approvals and the shift towards personalised medicine increases demand for datasets. The growing adoption of digital transformation, like e-health services, the internet, and others, supports the overall market growth.
Application Insights
How Drug Development and Approvals Segment Dominated the Real-World Data (RWD) Market?
The drug development and approvals segment dominated the market in 2024. The regulatory agencies, like EMA and FDA, increase drug development and approvals. The growing development of traditional drugs and focus on optimizing clinical trial designs increases demand for RWD. The growing therapeutic area complexity in areas like rare disease & oncology
The post-market surveillance segment is experiencing the fastest growth in the market during the forecast period. The strong focus on the detection of rare adverse reactions and long-term effects of drugs increases demand for post-market surveillance. The growing demand for increasing product effectiveness and enhancing safety features helps market growth. The availability of EHRs and the rise in digital health tools support the overall market growth.
End User Insights
Which End User Held the Largest Share in the Real-World Data (RWD) Market?
The pharmaceutical and medical device
The healthcare providers segment is the fastest-growing in the market during the forecast period. The strong focus on patient management and the growing development of personalised medicines requires healthcare providers. The increasing need to lower drug development delays and monitor disease progression requires healthcare providers. The high prevalence of chronic diseases and a strong focus on enhancing operational efficiency support the overall market growth.
✚ Related Topics You May Find Useful:
➡️ Real-World Evidence (RWE) Solutions Market: Explore how data-driven decision-making is transforming clinical approvals and regulatory pathways
➡️ Life Science Big Data Market: Uncover how AI and analytics are accelerating breakthroughs in genomics, drug discovery, and precision medicine
➡️ Clinical Data Management Market: Track how digital platforms and automation are enhancing trial efficiency and data accuracy worldwide
➡️ Synthetic Control Arms Market: Learn how RWD-powered virtual controls are reducing trial costs and reshaping evidence generation
➡️ Healthcare Data Monetization Solutions Market: See how health organizations are unlocking new revenue streams through secure, compliant data utilization
➡️ Health Economics and Outcomes Research (HEOR) Services Market: Understand how HEOR insights guide market access, pricing strategies, and value-based healthcare decisions
➡️ DNA Data Storage Market: Discover how DNA-based storage is emerging as the next frontier for ultra-long-term, high-density data archiving
Top Companies in the Real-World Data (RWD) Market & Their Offerings:
- Cerner Corporation: Offers access to de-identified EHR data via a secure, cloud-based platform for research. Evidera, Inc.: Provides consulting and studies using RWD to demonstrate drug effectiveness, safety, and value to regulators. Flatiron Health, Inc.: Specializes in high-quality, curated oncology datasets from EHRs for cancer research. IBM Corporation: Uses AI and software platforms to analyze RWD, integrating client data for predictive analytics. IQVIA Holdings Inc.: Combines extensive healthcare datasets, advanced analytics, and services to accelerate drug development with RWE. Optum, Inc.: Utilizes vast insurance claims and pharmacy data to provide RWE solutions that improve healthcare system efficiency. Palantir Technologies Inc.: Offers a software platform to help organizations integrate and manage their own RWD sources for unified analysis. SAS Institute Inc.: Provides analytics and AI software enabling stakeholders to manage and analyze diverse RWD sources for decision-making. Syneos Health Inc.: Offers integrated RWE services, using a data ecosystem to design and execute real-world and late-phase clinical studies. Tempus Labs Inc.: Focuses on precision medicine by building one of the largest libraries of clinical and molecular data for research.
Recent Developments in the Real-World Data (RWD) Industry
- In February 2025, CitiusTech launched a real-world data platform, CitiusTech HealthSPARX, for life sciences organizations. The platform offers accelerated insights and efficient management of data. The platform supports research, commercial, clinical, and medical operations. (Source: )
In January 2025, Carelon Research launched new real-world data, Carelon Real World Data, to support healthcare evidence generation. The data includes enrolment records since 2006, enhanced oncology data, pharmacy & medical closed claims, commercial & medicare populations, and laboratory results. (Source: )
In May 2024, HealthVerity collaborated with Castor to launch the platform, eConsent, to incorporate real-world data throughout the clinical trial lifecycle. The platform offers details about how patient RWD is used and is customizable. The platform contextualizes findings during the trial, conducts long-term follow-up, enhances eligibility screening, and continues monitoring of patients. (Source: )
Segments Covered in the Report
By Component
- Services Datasets
By Application
- Drug Development & Approvals Market Access & Reimbursement/Coverage Decisions Post-market Surveillance Clinical Research Other
By End User
- Pharmaceutical & Medical Device Companies Healthcare Payers Healthcare Providers Government Agencies Others
By Region
- North America
- U.S. Canada Mexico Rest of North America
- Brazil Argentina Rest of South America
- Western Europe
- Germany Italy France Netherlands Spain Portugal Belgium Ireland UK Iceland Switzerland Poland Rest of Western Europe
- Austria Russia & Belarus Türkiye Albania Rest of Eastern Europe
- China Taiwan India Japan Australia and New Zealand, ASEAN Countries (Singapore, Malaysia) South Korea Rest of APAC
- GCC Countries
- Saudi Arabia United Arab Emirates (UAE) Qatar Kuwait Oman Bahrain
Thank you for reading. You can also get individual chapter-wise sections or region-wise report versions, such as North America, Europe, or Asia Pacific.
Immediate Delivery Available | Buy This Premium Research Report@You can place an order or ask any questions, please feel free to contact at ... | +1 804 441 9344
Stay Ahead with Precedence Research Subscriptions
Unlock exclusive access to powerful market intelligence, real-time data, and forward-looking insights, tailored to your business. From trend tracking to competitive analysis, our subscription plans keep you informed, agile, and ahead of the curve.
Browse Our Subscription Plans@About Us
Precedence Research is a worldwide market research and consulting organization. We give an unmatched nature of offering to our customers present all around the globe across industry verticals. Precedence Research has expertise in giving deep-dive market insight along with market intelligence to our customers spread crosswise over various undertakings. We are obliged to serve our different client base present over the enterprises of medicinal services, healthcare, innovation, next-gen technologies, semi-conductors, chemicals, automotive, and aerospace & defense, among different ventures present globally.
Web:Our Trusted Data Partners:
Towards Healthcare | Towards Packaging | Towards Automotive | Towards Chem and Materials | Towards FnB | Towards Consumer Goods | Statifacts | Towards EV Solutions | Towards Dental | Nova One Advisor Market Stats Insight | Nutraceuticals Func Foods | Onco Quant | Sustainability Quant Specialty Chemicals AnalyticsGet Recent News:
/newsFor the Latest Update Follow Us:
LinkedIn | Medium | Facebook | Twitter
Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.

















Comments
No comment