
Indonesia jumps the gun on Ivermectin as Covid cure
(MENAFN- Asia Times) JAKARTA – Still under police investigation for the alleged illegal importation last year of the anti-parasitic drug Ivermectin to use as a potential weapon against the Covid-19 pandemic, philanthropist Haryoseno has learned a bitter lesson.“In this world,” he says,“to do good and help people isn't easy.”
As the owner of pharmaceutical firm Harsen Laboratories, Haryoseno's crime was to hand out free samples of Ivermectin, mostly to charitable groups, while he waited more than a year for the government to issue him with a license to manufacture and sell the drug.
In the meantime, with Covid-19 on the rampage, Ivermectin had become a hot seller on the black market after clinical trials in Australia and the United States showed that as effective as it was against parasites, it also helped prevent the virus from reproducing.
Doctors at state hospitals have already been prescribing the broad-spectrum drug and empirical evidence suggests it is now being widely used, even among directors in corporate boardrooms, both as a prophylactic and also at the first onset of the disease.
The jury is still out on Ivermectin's Covid efficacy and will be another five or six months before the Indonesian Health Ministry receives the results of its own trials reportedly being conducted among 10,000-20,000 patients in ten state-run hospitals.
The ministry has said little about the trials and there are already complaints about a lack of transparency, with the Food and Drugs Authority (BPOM) issuing conflicting responses to reports that it has been approved for emergency use.
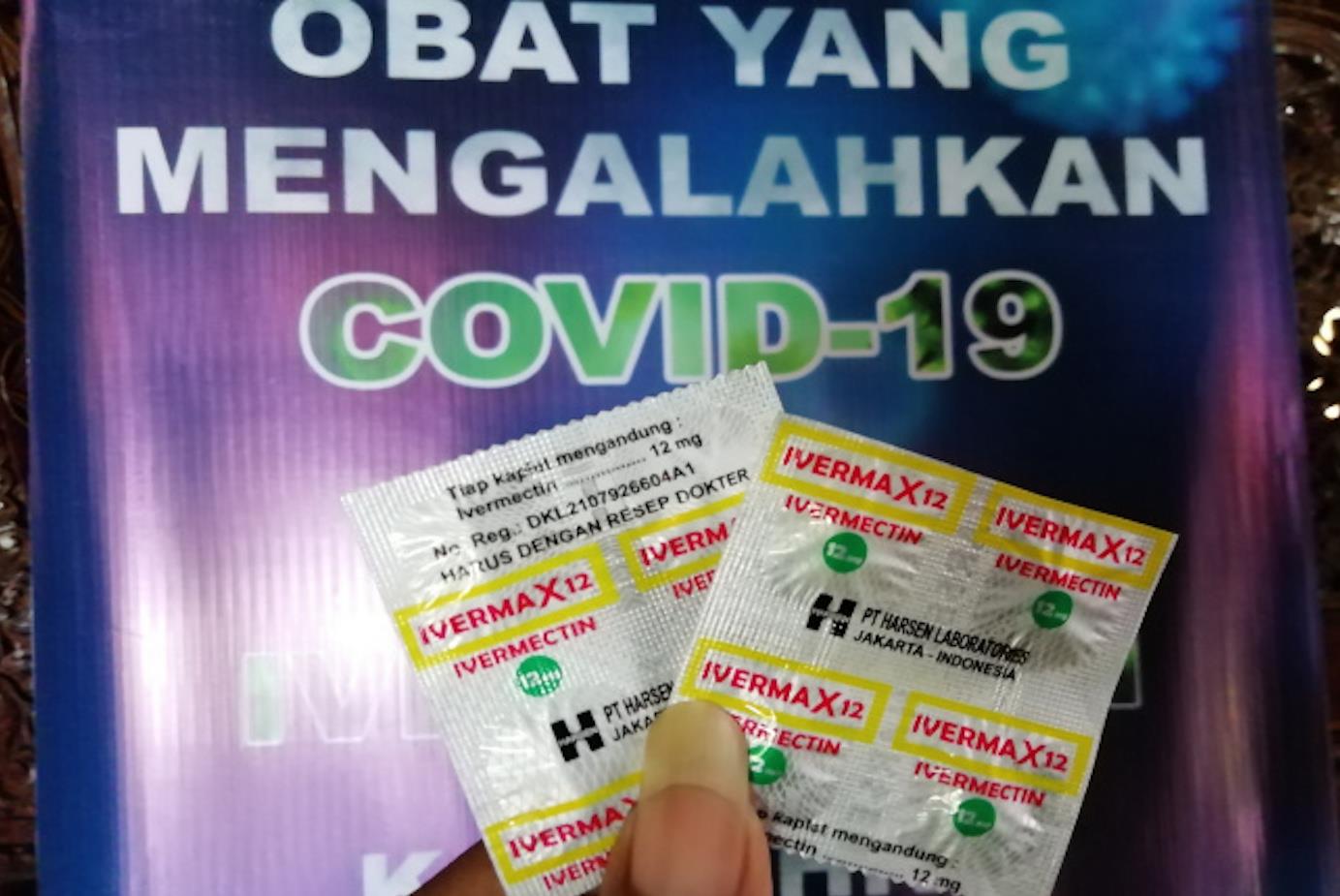
Ivermax 12 packets for sale in Indonesia. Image: Facebook
Meanwhile, Haryoseno has been compelled to apologize to BPOM over alleged claims made by three of its executives that the public was free to buy the tablets, marketed as Ivermax 12, without the need for a doctor's prescription or supervision.
In fact, imported black market Ivermectin was being sold over the counter as early as April 2020 when Harsen first applied for its license, an unregulated, self-medicating practice that applies to almost any drug sold by Indonesian pharmacies.
Based on BPOM allegations, Haryoseno is still facing criminal charges of illegally importing the raw materials for Ivermectin, distributing the drug without a permit, extending its expiration date and promoting it as an effective treatment against Covid-19.
Harsen was finally issued with its license to distribute Ivermectin as a parasitic drug on June 20 this year, but that was promptly withdrawn only a week later when BPOM officials seized its entire inventory of pills in a raid on a Jakarta warehouse.
What is not so well known is that state-owned pharmaceutical company PT Indofarma Tbk had been issued with a similar license a week before Harsen received its approval – and apparently without having to go through the same prolonged process.
Indofarma quickly announced plans to produce 4.5 million pills, even before the clinical trials had started. That has led to speculation that State Enterprise Minister Erick Thohir is in conflict with BPOM head Penny Lukito over whether to wait for the trial results.
Thohir and presidential chief of staff Moeldoko have publicly promoted Ivermectin as an effective treatment for the disease despite the World Health Organization (WHO) warning that it should only be deployed under certain clinical settings.

State Enterprise Minister Erick Thohir in a file photo. Image: Facebook
Spurred on by social media influencers – and a surge in new coronavirus infections that has made Indonesia the latest global epicenter of the disease – the drug has been flying off the shelves in recent weeks as a touted miracle cure for Covid victims isolating at home.
The non-profit monitoring organization LaporCovid has reported that 721 patients died in self-isolation between early June and July 21, in many cases because of overflowing hospital wards.
Worried about escalating prices, Thohir has cleared Kimia Farma, the state pharmacy chain, to sell limited supplies of the drug at 7,885 rupiah (54 cents) per 12-milligram tablet, compared to the average international price of $4.60.
Coordinating Minister for Maritime Affairs and Investment Luhut Panjaitan, who is in charge of the emergency measures introduced to counter an alarming rise in coronavirus cases, weighed into the debate by saying that there was nothing wrong with prescribing it for patients with minor symptoms.
“It works, so just go for it,” he said in a podcast interview, referring to advice he said he had received from a doctor at one state hospital.“This is an emergency. As long as it is good for the people and there is solid evidence, why not?”
But sources in the pharmaceutical industry say Indofarma does not have enough of the raw material that meets international Current Good Manufacturing Practice (CGMPs) standards and may have to wait months for fresh supplies before going into mass production.
Haryoseno says the government had informed him the suspension of his license will eventually be lifted, but as he told Asia Times:“We don't know when. It may be next month or it may be next year. We're hoping that the case will soon be resolved and that one day we can produce Ivermectin to treat Covid.”

Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.

















Comments
No comment