
EU Approves Gilead's New HIV Prevention Drug
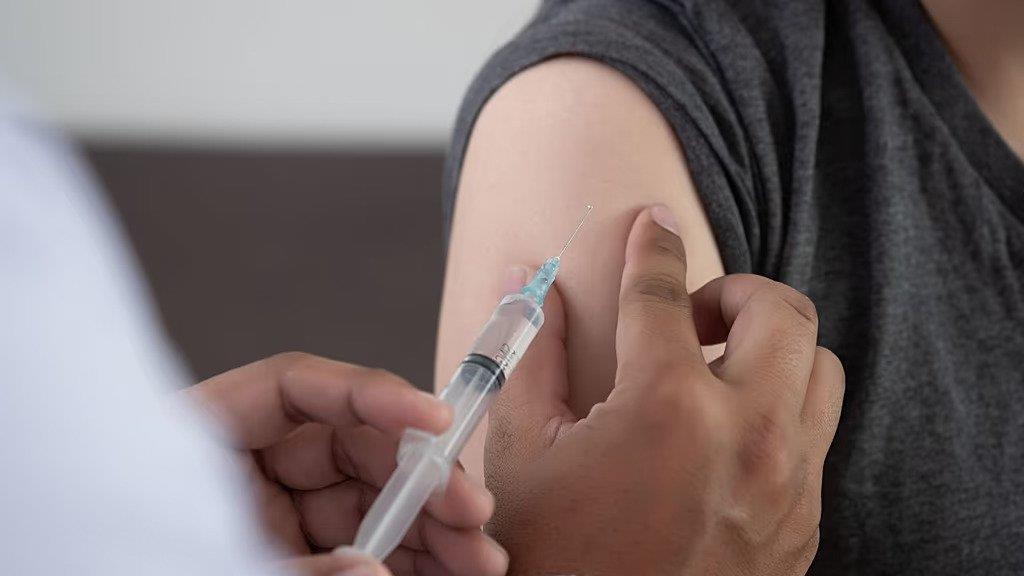
The European Commission has granted marketing authorization for Gilead Sciences' lenacapavir, a twice-yearly injection aimed at preventing HIV infection. Branded as Yeytuo in Europe, the drug is intended for pre-exposure prophylaxis (PrEP) to reduce the risk of sexually acquired HIV among high-risk adults and adolescents.
Lenacapavir has demonstrated nearly 100% effectiveness in large clinical trials, offering a long-acting alternative to daily oral medications. The World Health Organization recommended it as an additional HIV prevention option in July.
Gilead plans to collaborate with the Global Fund to make lenacapavir available to up to two million people in low-income countries over three years through royalty-free agreements with generic manufacturers. The company aims to address HIV prevention in regions with high infection rates.
In Europe, the availability of lenacapavir will depend on country-specific reimbursement negotiations. The drug's approval is expected to contribute to efforts in reducing HIV transmission rates across the continent.
While the U.S. list price for Yeztugo exceeds $28,000 annually, the European pricing structure will be determined through negotiations with national health systems. Analysts project the drug could achieve annual sales of over $4 billion by 2029.
The approval of lenacapavir marks a significant advancement in HIV prevention, providing individuals at high risk with a new option to protect against the virus.
As Gilead works to expand access to lenacapavir globally, its impact on HIV prevention strategies will be closely monitored by healthcare professionals and policymakers.
ShareFacebook Twitter WhatsApp Email Print Telegram
Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.

















Comments
No comment