
Japan Cell And Gene Therapy Market Set To Surge USD 2,016 Million By 2033: Trends & Outlook
Key Highlights
-
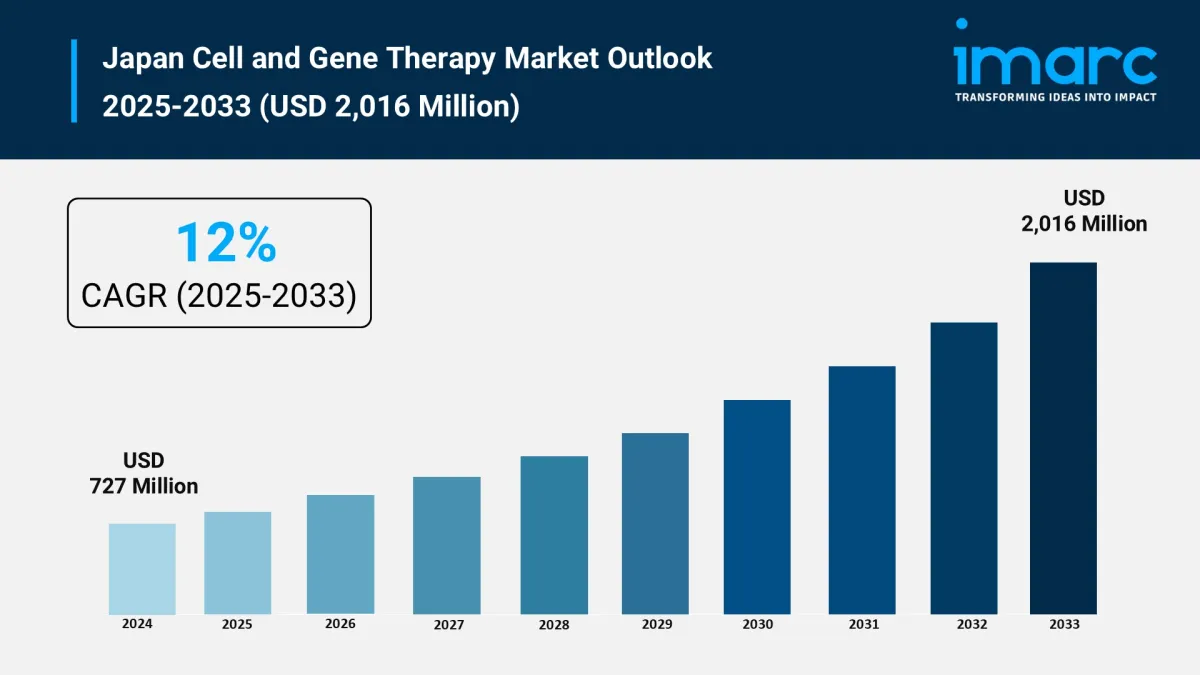
Market size (2024): USD 727 Million
Forecast (2033): USD 2,016 Million
CAGR (2025–2033): 12%
Gene-editing technologies, along with cell precision treatment, are rapidly gaining ground with greater attention to personal and regenerative medicine technologies.
Research grants, clinical trials, and commercialization programs are being fast-tracked with government funding, simplified regulations, and alliances with biotechnology companies in advanced therapies.
Autologous cell therapy owns the lion's share in the market, fueled by the wealth of applications in the field of oncology and rare genetic disorder treatments in Japan.
How Is AI Transforming the Cell and Gene Therapy Market in Japan?
-
Improvement in Regenerative Medicine: Deep learning that uses artificial intelligence (AI) to predict the quality of organoids with an accuracy of 70 percent reduces the number of errors and facilitates the development of personalized therapies in Japan.
Automation of Cell Production: Artificial intelligence-driven robotics automates iPSC manufacturing, giving advanced therapy developers the ability to produce high-volume batches, along with reducing the time-consuming aspects of advanced therapy manufacturing.
Driving Cost Reduction: Current government-funded AI automation efforts are addressing the cost of iPSC therapy, which is 80 to 200 times more expensive to administer (down to 1 million yen per case).
An accelerator of Clinical Innovation: The Kyoto University My iPS program applies AI-based systems to fully automated production of iPSCs, decreasing the timelines to cell-therapy production.
Groundbreaking AI-Guided Therapy: Osaka University achieves the first AI-assisted corneal stem-cell transplant in the world, creating a standard that will be used to complement the field of gene therapy and regenerative medicine.
Grab a sample PDF of this report: https://www.imarcgroup.com/japan-cell-gene-therapy-market/requestsample
Japan Cell and Gene Therapy Market Trends and Drivers
-
Expanding the Regenerative Medicine Use: Increasing pressure on the Japanese healthcare system due to demands for chronic diseases and rare genetic disease-related advanced therapies.
AI and Automation Integration: Intelligent systems of robotics and AI are automating PSC production and enhancing efficiency throughout the production and development of personalized therapy.
Growth of clinical research: The clinical trials that have increased because of academic-industry partnerships are meant to fast-track track commercialization of innovative therapeutic solutions.
Advances in gene editing: greater application of CRISPR and other precision treatments to develop specialized medication that treats genetic disorders and very rare diseases.
Government Support Programs: How funding, along with infrastructure building and short-track regulatory approval, can drive innovation and decrease time-to-market in the cell and gene therapy space.
Japan Cell and Gene Therapy Industry Segmentation:
The report has segmented the market into the following categories:
Analysis by Therapy Type:
-
Cell Therapy
-
Stem Cell
Non-Stem Cell
Analysis by Indication:
-
Cardiovascular Disease
Oncology Disorder
Genetic Disorder
Infectious Disease
Neurological Disorder
Others
Analysis by Delivery Mode:
-
In-Vivo
Ex-Vivo
Analysis by End User:
-
Hospitals
Cancer Care Centers
Pharmaceutical and Biotechnology Companies
Others
Regional Analysis:
-
Kanto Region
Kansai/Kinki Region
Central/ Chubu Region
Kyushu-Okinawa Region
Tohoku Region
Chugoku Region
Hokkaido Region
Shikoku Region
Competitive Landscape:
The competitive landscape of the industry has also been examined along with the profiles of the key players.
Recent News and Developments in Japan Cell and Gene Therapy Market
-
May 2025: Hitachi introduces its DesignCell platform, a new AI and robotics platform capable of designing and testing 100,000 CAR-T variants/yr to revolutionize the efficiency of cell therapy development.
June 2025: Cellares collaborates with Mitsui Fudosan to establish a standalone facility that will manufacture CAR-T treatment in Cellares-T Kashiwa City, which will run a commercial-level production that has 350 personnel.
August 2025: Fujifilm and HORIBA introduce a CEP (continuous electroporation) system to achieve a 100X increase in productivity of gene therapy manufacturing, relative to conventional gene delivery approaches.
Ask analyst of customized report: https://www.imarcgroup.com/request?type=report&id=17931&flag=E
Note: If you require specific details, data, or insights that are not currently included in the scope of this report, we are happy to accommodate your request. As part of our customization service, we will gather and provide the additional information you need, tailored to your specific requirements. Please let us know your exact needs, and we will ensure the report is updated accordingly to meet your expectations.
About Us:
IMARC Group is a global management consulting firm that helps the world's most ambitious changemakers to create a lasting impact. The company provides a comprehensive suite of market entry and expansion services. IMARC offerings include thorough market assessment, feasibility studies, company incorporation assistance, factory setup support, regulatory approvals and licensing navigation, branding, marketing and sales strategies, competitive landscape and benchmarking analyses, pricing and cost research, and procurement research.
Contact Us:
Street: 563-13 Kamien
Area: Iwata
Country: Tokyo, Japan
Postal Code: 4380111
Email: sales[@]imarcgroup.com
Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.

















Comments
No comment