
Sales Of Inguinal Hernia Mesh Devices Are Increasing Owing To Post-Operative Difficulties Associated With Traditional Surgical Approaches: Fact.MR X Herald
| Report Attributes | Details |
| Forecast Period | 2023 – 2033 |
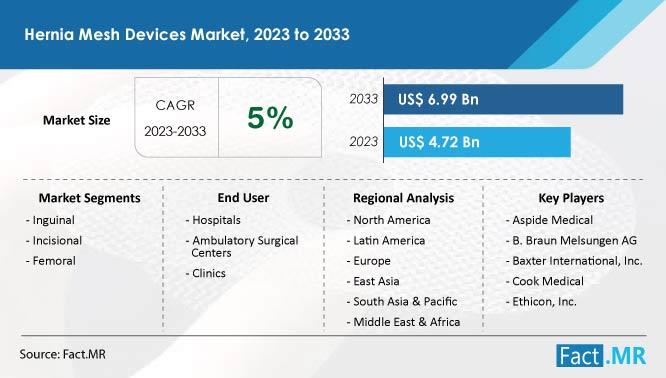
| Value Projection (2032) | US$ 6.99 Billion |
| Growth Rate (2022-2032) | 4 % CAGR |
| No. of Pages | 170 pages |
| No. of Tables | 80 Tables |
| No. of Figures | 227 Figures |
Key Takeaways from Market Study
- Global sales of hernia mesh devices are expected to reach US$ 6.99 billion by 2033. The market in China is predicted to expand rapidly at a CAGR of 6% during the projected period (2023 to 2033). Demand for hernia mesh devices for inguinal hernia treatment is expected to rise at a CAGR of 4% during the next 10 years. Sales of hernia mesh devices in Germany are anticipated to increase at a 3% CAGR.
“Increasing demand for advanced hernia mesh devices, rising adoption of robotic surgeries, and growing rate of the senior population are driving market growth,” says a Fact.MR analyst.
Recent Developments
- Becton, Dickinson, and Company gained FDA approval in July 2020 for their 3DMax MID Anatomical Mesh, which is intended for use in the repair of inguinal hernias and the strengthening of soft tissue. Deep Blue Medical Inc. obtained FDA approval in August 2020 to start selling its novel hernia mesh device.
As a result of the rising hernia incidence, hernia repair surgery rates are sharply rising. Due to the increasing demand for the strengthening and restoration of unhealthy hernia tissue, hernia repair devices are becoming increasingly popular throughout the healthcare industry.
Competitive Dashboard
Leading companies in the hernia mesh devices market are focusing on expanding their business on the regional level by changing pricing trends, improving product standards, quality control, and enhancing the local supply chain management system. Partnerships, collaborations, and new developments in products are also some of the marketing strategies adopted by market players.
- In March 2020, Novus Scientific AB got FDA clearance for TIGR Matrix Surgical Mesh, which is intended to be used in treatment methods such as soft tissue repairs, including hernias or other soft tissue-related abnormalities.
Market Titans
- Aspide Medical Atrium Braun Melsungen AG. Baxter International, Inc. R. Bard, Inc. Cook Medical Cousin Biotech Covidien (Part of Medtronic) Dipromed Ethicon, Inc. Feg Textiltechnik MBH Herniamesh S.r.l LifeCell Corporation L. Gore & Associates
Get Customization on this Report for Specific Research Solutions:
Industry Structure
· By Hernia Type :
- Inguinal Incisional Femoral
· By Mesh Type :
- Synthetic Biological
· By Procedure :
- Open Surgeries Laparoscopic Surgeries Robotic Surgeries
· By End-user :
- Hospitals Ambulatory Surgical Centers Clinics
· By Region :
- North America Latin America Europe East Asia South Asia & Oceania MEA
Key Questions Covered in the Hernia Mesh Devices Market Report
- What is the projected value of the Hernia Mesh Devices Market in 2023? At what rate will the global Hernia Mesh Devices Market grow until 2033? Which are the factors hampering the growth in the Hernia Mesh Devices Market? Which region is expected to lead in the global Hernia Mesh Devices Market during 2023 to 2033? Which are the factors driving the Hernia Mesh Devices Market during the forecast period? What is the expected market value of the Hernia Mesh Devices Market during the forecast period?
More Valuable Insights on Offer
Fact.MR, in its new offering, presents an unbiased analysis of the global hernia mesh devices market, presenting historical demand data (2018 to 2022) and forecast statistics for the period of 2023 to 2033.
The study divulges essential insights on the market on the basis of hernia type (inguinal, incisional, femoral), mesh type (synthetic, biological), procedure (open surgeries, laparoscopic surgeries, robotic surgeries), and end user (hospitals, ambulatory surgical centers, clinics), across five major regions of the world
Check out more related studies published by Fact.MR Research:
Contact:
US Sales Office :
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Tel: +1 (628) 251-1583
E-Mail:

Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.

















Comments
No comment