
Free-Radical Astrocyte Damage Linked To Dementia

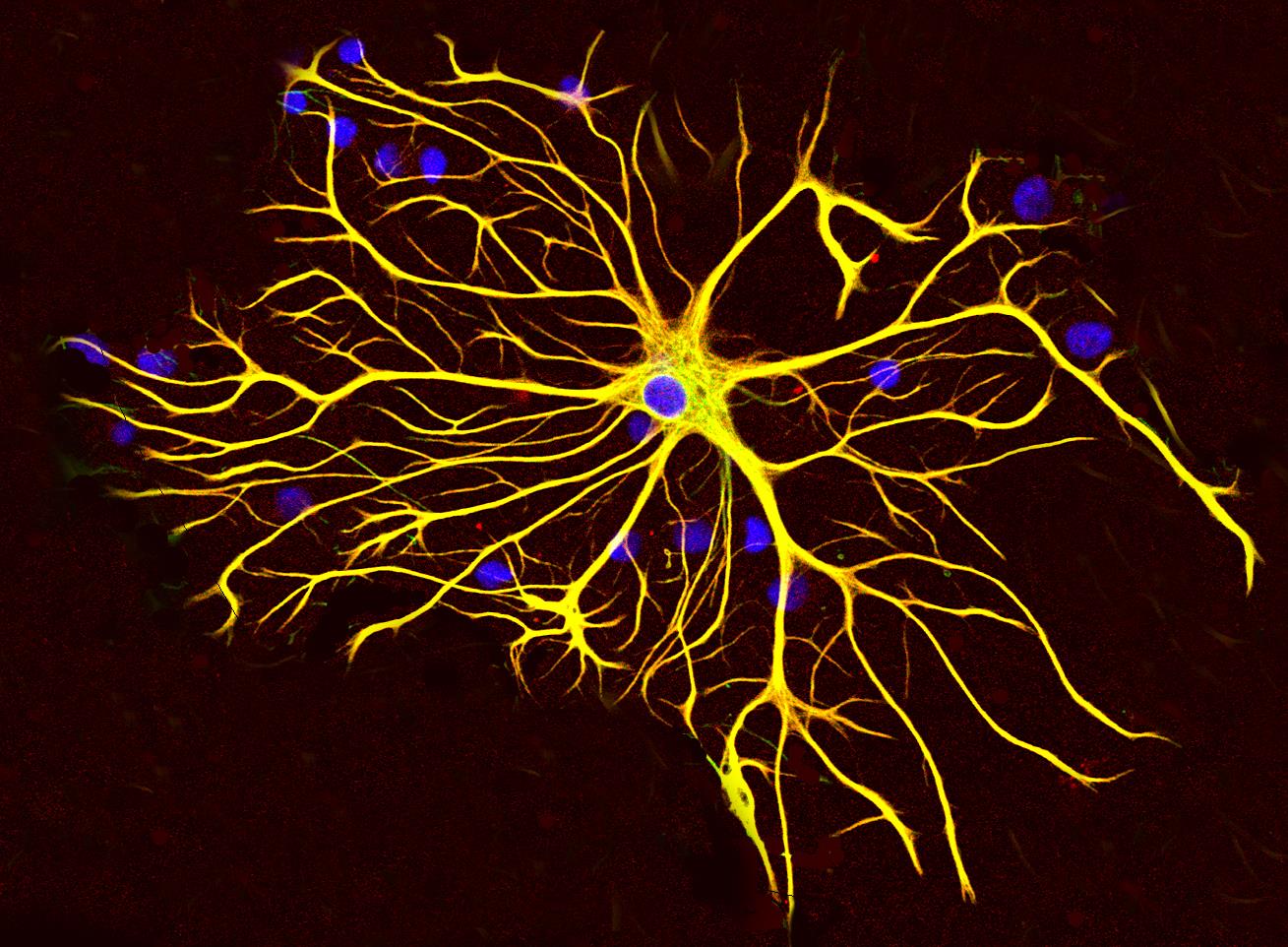
Researchers at Weill Cornell Medicine have identified a previously unrecognised source of oxidative stress in the brain that appears to drive neurodegeneration in disorders such as Alzheimer's disease and Frontotemporal dementia. Their study highlights the role of support cells known as astrocytes in generating excessive mitochondrial reactive oxygen species at a specific site - mitochondrial Complex III - and suggests that targeting this mechanism may open new therapeutic pathways.
The investigation found that astrocytic mitochondria leaking ROS via Complex III set off a cascade of immune-metabolic changes and neuronal damage. The research team, led by Dr Anna G. Orr and Dr Adam L. Orr, demonstrated that compounds called S3QELs, which selectively inhibit ROS production at the Complex III site, reduced astrocyte-driven inflammation and tau-linked pathology in mouse models of FTD. The treatment also extended lifespan in those mice, without apparent adverse effects.
Astrocytes, long considered auxiliary to neurons, are now taking centre stage in this work. The study shows that when astrocytes are exposed to disease-linked factors such as amyloid-β or inflammatory cytokines, their Complex III-derived ROS production increases markedly. That surge triggers oxidation of immune and metabolic proteins, alters the expression of thousands of genes, and promotes neuroinflammatory gene networks. This process undermines neighbouring neurons. Conventional antioxidant therapies have failed in the past because they could not precisely target the mitochondrial ROS source.
In their cell-culture experiments the authors observed that neuronal protection by S3QELs occurred only in the presence of astrocytes, underlining the cell-type specificity of the mechanism. They then verified the effect in an FTD mouse model: applying S3QELs after symptom onset reduced astrocyte activation, lowered inflammatory gene expression and decreased tau modification associated with neuronal damage. The authors emphasised that the approach preserved normal mitochondrial ATP generation while blocking only the detrimental ROS.
See also Solar Plasma“Rain” Explained by Shifting Elemental MixThe study's authors argue that this mechanism may reshape the understanding of how glial-neuronal interactions and mitochondrial dysfunction combine in dementia. Dr Anna Orr said that the precision of the mechanism“had not been previously appreciated” and now offers“a very nuanced process” to target. The results suggests that astrocytes may actively contribute to disease-driving oxidative stress, not merely respond to neuronal injury.
Major implications emerge: first, this work shifts focus away from neurons as the sole originators of ROS in neurodegeneration and puts astrocytes in view. Second, it offers a potential therapeutic target for diseases for which current treatments are limited. Third, it highlights the importance of mitochondrial sub-compartment targeting in drug development, rather than broad antioxidant approaches that have failed to translate clinically.
Caution remains. While the mouse-model results are promising, translation to human trials may pose significant challenges including verification of target engagement in humans, long-term safety and identification of patients who would benefit. Questions also remain about how genetic variants associated with dementia risk might influence astrocytic ROS production, and whether human astrocytes behave identically to those in mice. The research team indicated plans to investigate how disease-associated genes modulate mitochondrial ROS generation in astrocytes, and how that relates to risk in human populations.
This development arrives amid broader shifts in dementia research, where the role of non-neuronal cells and metabolic pathways is gaining traction. For instance, altered glial-neuron signalling, neuroinflammation, and mitochondrial dysregulation have become recognised as key components of neurodegenerative disease. The Orr Laboratory's work sits at the intersection of these domains and suggests that therapeutic strategies aimed at astrocytes and their mitochondria could be vital.
See also Jane Goodall: A Life Among Chimpanzees and Hope for Our PlanetNotice an issue? Arabian Post strives to deliver the most accurate and reliable information to its readers. If you believe you have identified an error or inconsistency in this article, please don't hesitate to contact our editorial team at editor[at]thearabianpost[dot]com. We are committed to promptly addressing any concerns and ensuring the highest level of journalistic integrity.
Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.

















Comments
No comment