
Medical Device Sterilization Market To Hit USD 28.93 Billion By 2033 Rising Demand For Infection Control In Healthcare To Augment Market Expansion SNS Insider
| Report Attributes | Details |
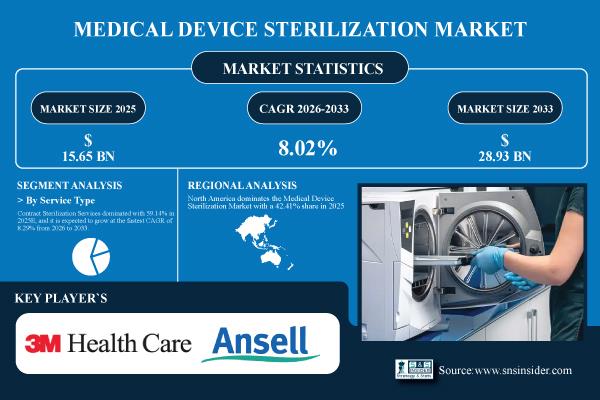
| Market Size in 2025E | USD 15.65 Billion |
| Market Size by 2033 | USD 28.93 Billion |
| CAGR | CAGR of 8.02% From 2026 to 2033 |
| Base Year | 2025E |
| Forecast Period | 2026-2033 |
| Historical Data | 2022-2024 |
| Key Segments | . By Sterilization Technique (Steam Sterilization (Autoclaving), Ethylene Oxide (EO) Sterilization, Radiation Sterilization (Gamma, E-Beam, X-ray), Hydrogen Peroxide Gas Plasma Sterilization, and Other Techniques (Ozone, Dry Heat, Peracetic Acid)) . By Device Type (Surgical Instruments, Implants, Diagnostic Equipment, Disposable Medical Devices, and Endoscopic Devices) . By Service Type (Contract Sterilization Services, and In-house Sterilization Services) . By End-User (Hospitals & Clinics, Ambulatory Surgical Centers, Diagnostic Laboratories, Medical Device Manufacturers, and Research & Academic Institutes) |
| Regional Analysis/Coverage | North America (US, Canada), Europe (Germany, UK, France, Italy, Spain, Russia, Poland, Rest of Europe), Asia Pacific (China, India, Japan, South Korea, Australia, ASEAN Countries, Rest of Asia Pacific), Middle East & Africa (UAE, Saudi Arabia, Qatar, South Africa, Rest of Middle East & Africa), Latin America (Brazil, Argentina, Mexico, Colombia, Rest of Latin America). |
Segmentation Analysis:
By Sterilization Technique
Steam Sterilization (Autoclaving) dominated with 34.86% in 2025E due to its proven reliability, cost-effectiveness, and widespread use for a variety of reusable medical instruments. Hydrogen Peroxide Gas Plasma Sterilization is expected to grow at the fastest CAGR of 9.95% from 2026 to 2033 driven by increasing demand for low-temperature, eco-friendly sterilization methods.
By Device Type
Surgical Instruments dominated with 31.57% in 2025E due to their high usage in healthcare settings, frequent reuse, and critical need for stringent sterilization to ensure patient safety. Disposable Medical Devices is expected to grow at the fastest CAGR of 9.25% from 2026 to 2033 driven by increasing demand for single-use products to reduce infection risks, rising awareness of hygiene, and advancements in cost-effective manufacturing.
By Service Type
Contract Sterilization Services dominated with 59.14% in 2025E and it is expected to grow at the fastest CAGR of 8.29% from 2026 to 2033 due to cost efficiency, access to advanced technologies, and scalability for manufacturers and healthcare providers.
By End-User
Hospitals & Clinics dominated with 49.33% in 2025E driven by the high demand for sterile instruments and strict infection control protocols. Medical Device Manufacturers is expected to grow at the fastest CAGR of 8.97% from 2026 to 2033 fueled by rising production of advanced medical devices, growing outsourcing of sterilization services, and the need for compliance with global sterilization standards across the healthcare industry.
Regional Insights:
North America dominates the Medical Device Sterilization Market with a 42.41% share in 2025E, driven by advanced healthcare infrastructure, stringent regulatory standards, and high adoption of sterilization technologies. The region's well-established hospitals, growing surgical procedures, and rising focus on patient safety further bolster demand.
Asia Pacific is expected to grow at the fastest CAGR of 8.86% from 2026–2033 in the Medical Device Sterilization Market. This growth is driven by expanding healthcare infrastructure, rising medical tourism, and increasing surgical procedures.
Recent Developments:
- In March 2025, STERIS Applied Sterilization Technologies announced the addition of Extractables and Leachables (E&L) testing, featuring advanced chemical analysis. In May 2025, Sterigenics announced the addition of a new X-ray sterilization facility in Haw River, North Carolina. This facility enhances their existing global sterilization platform, providing high-throughput processing for medical devices, pharmaceuticals, and biopharmaceuticals.
Exclusive Sections of the Report (The USPs):
- STERILITY PERFORMANCE INDEX – helps you assess the average Sterility Assurance Level (SAL) and cycle efficiency across major sterilization methods (EO, gamma, steam, H2O2), providing insight into reliability and safety benchmarks. TECHNOLOGY UTILIZATION METRICS – helps you understand adoption trends and utilization factors of different sterilization technologies, highlighting regions with higher contract vs. in-house sterilization penetration. QUALITY & COMPLIANCE BENCHMARKS – helps you track adherence to ISO 11135/11137/17665, FDA, and MDR standards, along with biological indicator failure rates and rework statistics to ensure process consistency. COST & OPERATIONAL EFFICIENCY ANALYSIS – helps you compare sterilization costs, throughput rates, and maintenance performance across methods to identify the most cost-efficient sterilization solutions. SUSTAINABILITY & ENERGY IMPACT METRICS – helps you measure energy consumption and carbon footprint (CO2e per cycle) across sterilization technologies, supporting ESG and green compliance goals. PROCESS TURNAROUND INSIGHTS – helps you evaluate average cycle and time-to-release durations, revealing workflow efficiency and throughput optimization opportunities for healthcare facilities and contract providers.
[For more information or need any customization research mail us at...]
About Us:
SNS Insider is one of the leading market research and consulting agencies that dominates the market research industry globally. Our company's aim is to give clients the knowledge they require in order to function in changing circumstances. In order to give you current, accurate market data, consumer insights, and opinions so that you can make decisions with confidence, we employ a variety of techniques, including surveys, video talks, and focus groups around the world.
CONTACT: Contact Us: Rohan Jadhav - Principal Consultant Phone: +1-315 636 4242 (US) | +44- 20 3290 5010 (UK) Email:...
Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.















Comments
No comment