
Solid Biosciences Reports Third Quarter 2025 Financial Results And Provides Update On INSPIRE DUCHENNE Clinical Trial Progress And Planned Regulatory Discussions
| Correlation of SGT-003 Microdystrophin Levels with Biomarker % Increase from Baseline (N=10) | Pearson Correlation* |
| Day 90 SGT-003 microdystrophin positive fibers and beta-sarcoglycan positive fibers | 0.95 |
| Day 90 SGT-003 microdystrophin positive fibers and nNOS activity | 0.95 |
| Correlation of SGT-003 Microdystrophin Levels with Biomarker % Decrease from Baseline (N=7 unless noted) | Pearson Correlation** |
| Day 90 SGT-003 microdystrophin expression (mass spectrometry) and Day 180 CK | -0.78 |
| Day 90 SGT-003 microdystrophin expression (western blot) and Day 180 CK | -0.71 |
| Day 90 SGT-003 microdystrophin positive fibers (immunofluorescence) and Day 180 CK | -0.54 |
| Day 90 SGT-003 microdystrophin expression (western blot) and Day 180 LDH | -0.71 |
| Day 90 SGT-003 microdystrophin expression (mass spectrometry) and Day 180 LDH | -0.55 |
| Day 90 SGT-003 microdystrophin expression (western blot) and Day 180 AST | -0.54 |
| Day 90 SGT-003 microdystrophin positive fibers and embryonic myosin heavy chain (eMHC) positive fibers (N=10) | -0.51 |
| *A score of 1 indicates a perfect, positive linear relationship; **A score of -1 indicates a perfect, negative linear relationship; Larger absolute values indicate stronger correlations. |
SGT-003, utilizing the Company's proprietary, rationally designed capsid, AAV-SLB101, has demonstrated strong transduction, achieving a mean of 13 vector copies per nucleus (N=10) at Day 90, along with meaningful restoration of biologic correlates across several measures of microdystrophin, components of the DAPC, and multiple biomarkers of muscle integrity and preservation.
In the 10 participants (aged 5-10) whose Day 90 biopsies were evaluated as of the September 29, 2025, data cutoff date, the Company observed mean microdystrophin expression of 58%, as measured by both western blot and mass spectrometry, and mean microdystrophin positive fibers of 51%, as measured by immunofluorescence. Furthermore, in each of those 10 participants, the Company observed properly localized and restored beta-sarcoglycan positive fibers at the mean 50% level as measured by immunofluorescence and nNOS activity-positive fibers (a less sensitive activity assay) at the mean 26% level.
Available Day 360 biopsy data from 2 participants (aged 5) as of September 29, 2025, demonstrated encouraging and durable transduction, achieving a mean of 12 vector copies per nucleus, as well as robust mean microdystrophin expression of 107%, as measured by western blot, and 100%, as measured by mass spectrometry, mean microdystrophin positive fibers of 67% and mean beta-sarcoglycan positive fibers of 70%, both measured by immunofluorescence, and mean nNOS activity-positive fibers of 36%.
Additionally, a mean 49% reduction in percent eMHC positive fibers, a histologic marker of muscle regeneration and disease progression, was observed at Day 90 (N=10). As muscle fibers deteriorate, muscle stem cells are activated to repair and replace damaged muscle fibers; during this process, new muscle fibers transiently express eMHC. In Duchenne, this stem cell-mediated repair process is futile because muscle fibers that are developed from stem cells lack dystrophin and therefore will be dystrophic. Consequently, the presence of eMHC positive fibers is an informative biomarker of disease progression, signaling constant muscle injury, breakdown and deterioration. A treatment-mediated decrease in eMHC is a favorable observation, and in combination with other markers of reduced muscle injury, suggests overall muscle preservation.
Favorable reductions across a range of biomarkers of muscle injury and breakdown were observed through both Day 90 and Day 360:
| Serum Biomarkers | Day 90 Mean Reductions (N=14 unless noted) | Day 360 Mean Reductions (N=3 unless noted) |
| Serum creatine kinase (CK) | 34% | 42% |
| Serum alanine transaminase (ALT) | 41% | 29% |
| Serum aspartate aminotransferase (AST) | 25% | 40% |
| Serum lactate dehydrogenase (LDH)* | 42% | 46% |
| Serum titin** | 22% | 25% |
| *N=12 participant samples available at Day 90 for LDH (two samples hemolyzed); **N=11 participant samples available at Day 90 and N=2 samples available at Day 360 for titin, which was batch-analyzed at an earlier cutoff date. |
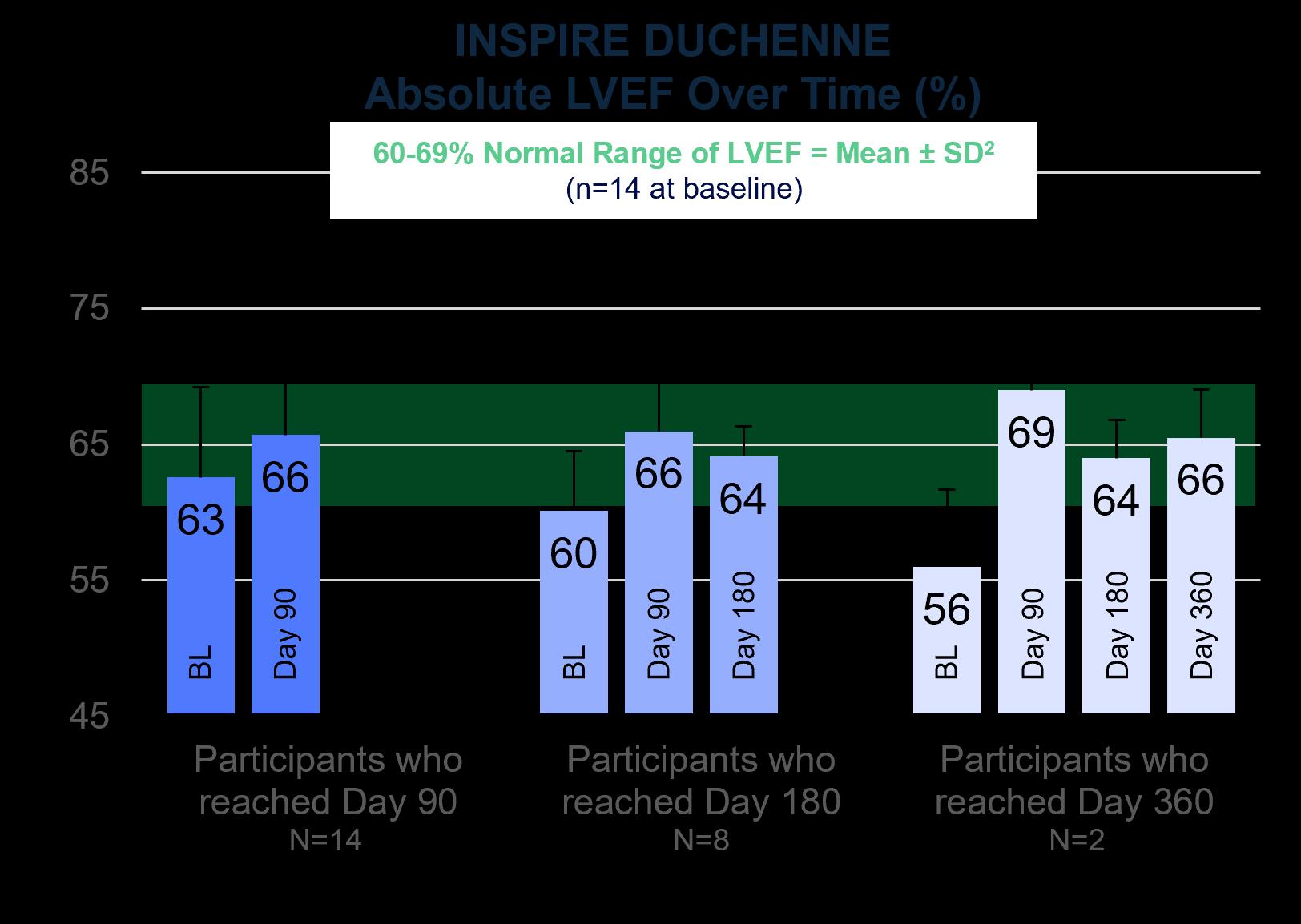
INSPIRE DUCHENNE – Interim Cardiac Monitoring
Cardiomyopathy is a leading cause of death in Duchenne, with 25% of individuals displaying evidence of cardiomyopathy by six years of age, increasing to 59% by 10 years of age.1
Mean cardiac function trended into normal LVEF ranges (60-69%)2 for all SGT-003-treated participants who reached the Day 180 follow-up timepoint (N=8) as of the September 29, 2025, data cutoff date. Though cardiac injury biomarkers and cardiac imaging were collected primarily for safety analysis, early data may indicate a potential for benefit through reduction in troponin I (cTnI) and increased systolic function as measured by LVEF by echocardiography. Observed increases in systolic function as measured by LVEF appeared to have been driven largely by participants with low to low-normal systolic function at baseline.
Mean reductions from baseline in serum cTnI of 31% at Day 90 (N=14) and 70% at Day 360 (N=3) were observed with reductions driven by participants who entered the trial with elevated baseline cTnI levels. cTnI is an important marker that can be predictive of severe cardiac disease in neuromuscular diagnoses.
INSPIRE DUCHENNE – Interim Safety Update
SGT-003 has been generally well tolerated in the 23 participants dosed as of October 31, 2025. Steroids alone were utilized as the prophylactic immunomodulation regimen. Signals of asymptomatic and self-resolving platelet declines and thrombocytopenia observed in early participants in the trial have been ameliorated in subsequent participants.
As of October 31, 2025, there was one treatment-related serious adverse event (SAE) reported in the INSPIRE DUCHENNE trial. This SAE was identified as a Grade 3 immune-mediated myositis which, importantly, was not associated with muscle pain or weakness, and occurred in a participant who had a large deletion in a region coded for by SGT-003's microdystrophin. The participant promptly responded to steroid treatment with all clinical symptoms noted at presentation resolving and with muscle biomarkers, including CK, declining well below baseline levels. This SAE was reviewed by the trial data and safety monitoring board (DSMB) with the recommendation to continue dosing without interruption.
In Duchenne muscular dystrophy, transaminase elevations are the result of ongoing muscle injury as opposed to liver injury. Therapeutic interventions that lead to reductions in transaminases therefore indicate muscle protection in the setting of an avoidance of demonstrable liver injury, especially when more specific liver injury markers remain stable. As of the September 29, 2025, data cutoff date, we observed a mean alanine transaminase (ALT) reduction of 41% (N=14), a mean aspartate transaminase (AST) reduction of 25% (N=14) and stable mean gamma-glutamyl transferase (GGT) levels through Day 90 (N=14). Mean reductions of 40% AST and 29% ALT were observed in the three participants who reached the Day 360 follow-up.
There have been no cases of drug-induced liver injury (DILI) observed as of October 31, 2025 (N=23).
A presentation summarizing the interim data update can be accessed on the Presentations page of the Investors section of the Company's website.
SGT-003 Regulatory Update
Solid plans to meet with the FDA in the first half of 2026 to discuss potential registrational pathways, including accelerated approval pathways, for SGT-003. Solid continues to dose participants in the INSPIRE DUCHENNE trial in the interim, with additional participant safety, clinical activity and functional data expected to enable a more robust discussion with the FDA.
Critically, Solid has aligned with the FDA on SGT-003's potency assay strategy and will continue additional commercial-readiness CMC activities, with PPQ manufacturing batches expected to be completed in 2026.
In October 2025, Solid activated the first clinical trial site and began participant screening for IMPACT DUCHENNE, a Phase 3 randomized, double-blind, placebo-controlled clinical trial assessing SGT-003. IMPACT DUCHENNE will be conducted in pediatric participants outside of the United States (U.S.) and was designed to support potential ex-U.S. regulatory authorizations. We have received regulatory approvals to conduct IMPACT DUCHENNE in both Canada and Australia, and we plan to expand the trial into additional countries, subject to receipt of regulatory approvals.
SGT-212 for Friedreich's Ataxia (FA)
In October 2025, the Company activated the first clinical trial site and began participant screening for FALCON, a first-in-human, open-label, Phase 1b clinical trial of SGT-212. The trial is expected to enroll non-ambulatory and ambulatory adult participants living with FA in up to three cohorts and is designed to evaluate the safety and tolerability of systemic and bilateral intradentate nucleus (IDN) administration of SGT-212.
SGT-212 is the first investigational gene therapy for FA to utilize a dual route of administration and is intended to promote restoration of therapeutic levels of the frataxin protein to address the neurologic, cardiac and systemic clinical manifestations of FA.
SGT-501 for Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT)
In the fourth quarter of 2025, Solid expects to activate the first clinical trial site for ARTEMIS, a first-in-human, open-label, Phase 1b clinical trial of SGT-501. The trial is expected to enroll adult participants with CPVT and is designed to evaluate the safety, tolerability and efficacy of SGT-501.
SGT-501 is a novel gene therapy candidate intended to promote excess levels of the cardiac CASQ2 protein to address the underlying ryanodine receptor (RYR2) instability and calcium dysregulation seen in CPVT. There are currently no approved treatments that address the underlying mechanisms of CPVT.
Platform Technologies – Capsids
AAV-SLB101, the Company's proprietary, next-generation capsid used in SGT-003, has been generally well tolerated in the 23 participants dosed in the INSPIRE DUCHENNE trial as of October 31, 2025, and has shown compelling levels of vector transduction, protein expression, and reduced liver targeting.
Solid has executed over 30 agreements, including licenses, with corporations, institutions and academic labs for the use of AAV-SLB101, with additional agreements and licenses expected to be executed by year end.
Additionally, the Company is building multiple cardiac and neuromuscular next-generation capsid and promoter libraries with final capsid selection from the first cardiac capsid library anticipated in the first half of 2026.
Third Quarter 2025 Financial Highlights
- Cash Position: Solid had $236.1 million in cash, cash equivalents, and available-for-sale securities as of September 30, 2025, compared to $148.9 million as of December 31, 2024. The Company expects that its cash, cash equivalents, and available-for-sale securities as of September 30, 2025, will enable it to fund its operational runway into the first half of 2027.
Research and Development (R&D) Expenses: R&D expenses for the third quarter of 2025 were $38.9 million, compared to $27.3 million for the third quarter of 2024. The increase of $11.5 million in research and development expenses was primarily due to a $12.8 million increase in costs for SGT-003 primarily related to manufacturing, regulatory, and clinical costs, a $2.7 million increase in personnel related expenses, a $0.9 million increase in costs for SGT-601 primarily related to manufacturing costs and research costs, partially offset by a $3.3 million decrease in costs for SGT-212 primarily related to lower license and milestone related costs partially offset by an increase in clinical costs, and a $1.8 million decrease in costs for SGT-501 primarily related to lower research and manufacturing costs.
General and Administrative (G&A) Expenses: G&A expenses for the third quarter of 2025 were $9.2 million, compared to $7.9 million for the third quarter of 2024. The increase of $1.3 million was primarily related to a $0.9 million increase in personnel-related costs and a $0.4 million increase in legal and consulting fees.
Net Loss: Net loss for the third quarter of 2025 was $45.8 million, compared to $32.7 million for the third quarter of 2024.
References:
1. Gandhi S, et al. Cells. 2024;13(14):1168.
2. Romanowicz J, et al. J Am Soc Echocardiogr. 2023;36(3):310-323.
About Duchenne
Duchenne is a genetic muscle-wasting disease predominantly affecting boys, with symptoms usually appearing between three and five years of age. Duchenne is a progressive, irreversible, and ultimately fatal disease that affects approximately one in every 3,500 to 5,000 live male births and has an estimated prevalence of 5,000 to 15,000 cases in the United States alone.
About SGT-003
SGT-003 is an investigational gene therapy containing a differentiated microdystrophin construct and a proprietary, next-generation capsid, AAV-SLB101, which was rationally designed to target integrin receptors, and has shown enhanced cardiac and skeletal muscle transduction with decreased liver targeting in nonclinical studies. SGT-003's microdystrophin construct uniquely includes the R16/17 binding domain, which localizes nNOS to the muscle membrane. Nonclinical studies have shown that nNOS can improve blood flow to the muscle thereby reducing muscle breakdown from ischemia and muscle fatigue. Together, these design features suggest that SGT-003 could be a potential best-in-class investigational gene therapy for the treatment of Duchenne.
About INSPIRE DUCHENNE
INSPIRE DUCHENNE is a first-in-human, open-label, single-dose, multicenter Phase 1/2 clinical trial to evaluate the safety, tolerability and efficacy of SGT-003 in pediatric participants with a genetically confirmed Duchenne diagnosis with a documented dystrophin gene mutation. INSPIRE DUCHENNE is a multinational trial designed to enroll participants in the United States, Canada, the United Kingdom and Italy.
About IMPACT DUCHENNE
IMPACT DUCHENNE is a Phase 3 randomized, double-blind, placebo-controlled trial to evaluate the efficacy of a single dose of SGT-003 in pediatric participants with a genetically confirmed Duchenne diagnosis with a documented dystrophin gene mutation. IMPACT DUCHENNE is a multinational trial designed to enroll participants outside of the United States with the aim of supporting potential ex-U.S. regulatory authorizations.
About Solid Biosciences
Solid Biosciences is a precision genetic medicine company focused on advancing a portfolio of gene therapy candidates targeting rare neuromuscular and cardiac diseases, including SGT-003 for Duchenne muscular dystrophy (Duchenne), SGT-212 for Friedreich's ataxia (FA), SGT-501 for catecholaminergic polymorphic ventricular tachycardia (CPVT), SGT-601 for TNNT2-mediated dilated cardiomyopathy and additional fatal, genetic cardiac diseases. The Company is also focused on developing innovative libraries of genetic regulators and other enabling technologies with promising potential to significantly impact gene therapy delivery cross-industry. Solid is advancing its diverse pipeline and delivery platform in the pursuit of uniting experts in science, technology, disease management, and care. Patient-focused and founded by those directly impacted by Duchenne, Solid's mission is to improve the daily lives of patients living with devastating rare diseases. For more information, please visit .
Cautionary Note Regarding Forward-Looking Statements
This press release contains“forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including statements regarding future expectations, plans and prospects for the company; the ability to successfully achieve and execute on the company's goals, priorities and key clinical and preclinical milestones; strategies and expectations for the company's SGT-003, SGT-212, SGT-501 and SGT-601 programs; expectations for additional site activations, planned enrollment, planned regulatory interactions and the potential approval pathways for SGT-003; plans for enrollment in the clinical trial of SGT-212; timing of planned clinical trial of SGT-501; the cash runway of the company and the sufficiency of the Company's cash, cash equivalents, and available-for-sale securities to fund its operations; and other statements containing the words“anticipate,”“believe,”“continue,”“could,”“estimate,”“expect,”“intend,”“may,”“plan,”“potential,”“predict,”“project,”“should,”“target,”“would,”“working” and similar expressions. Any forward-looking statements are based on management's current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in, or implied by, such forward-looking statements. These risks and uncertainties include, but are not limited to, risks associated with the company's ability to advance SGT-003, SGT-212, SGT-501, SGT-601 and other preclinical programs, capsid libraries and other enabling technologies on the timelines expected or at all; obtain and maintain necessary approvals from the FDA and other regulatory authorities; replicate in clinical trials positive results found in preclinical studies and early-stage clinical trials of the company's product candidates; obtain, maintain or protect intellectual property rights related to its product candidates; replicate preliminary or interim data from clinicals trials in the final data of such trials; compete successfully with other companies that are seeking to develop Duchenne, FA, CPVT and other neuromuscular and cardiac treatments and gene therapies; manage expenses; and raise the substantial additional capital needed, on the timeline necessary, to continue development of SGT-003, SGT-212, SGT-501, SGT-601 and other candidates, achieve its other business objectives and continue as a going concern. For a discussion of other risks and uncertainties, and other important factors, any of which could cause the company's actual results to differ from those contained in the forward-looking statements, see the“Risk Factors” section, as well as discussions of potential risks, uncertainties and other important factors, in the company's most recent filings with the Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent the company's views as of the date hereof and should not be relied upon as representing the company's views as of any date subsequent to the date hereof. The company anticipates that subsequent events and developments will cause the company's views to change. However, while the company may elect to update these forward-looking statements at some point in the future, the company specifically disclaims any obligation to do so.
Solid Biosciences Investor Contact:
Nicole Anderson
Director, Investor Relations and Corporate Communications
Solid Biosciences Inc.
...
Media Contact:
Glenn Silver
FINN Partners
...
| SELECTED FINANCIAL INFORMATION (UNAUDITED) | |||||
| CONDENSED CONSOLIDATED BALANCE SHEETS | September 30, | December 31, | |||
| (in thousands, except share data) | 2025 | 2024 | |||
| Cash and cash equivalents | $ | 61,364 | $ | 80,235 | |
| Available-for-sale securities | 174,778 | 68,685 | |||
| Prepaid expenses and other current assets | 8,710 | 8,382 | |||
| Operating lease, right-of-use assets | 22,535 | 24,295 | |||
| Property and equipment, net | 4,356 | 4,747 | |||
| Other non-current assets | 247 | 366 | |||
| Restricted cash | 1,924 | 1,952 | |||
| Total Assets | $ | 273,914 | $ | 188,662 | |
| Accounts payable | $ | 8,429 | $ | 4,237 | |
| Accrued expenses and other current liabilities | 19,050 | 19,852 | |||
| Operating lease liabilities | 2,032 | 1,787 | |||
| Finance lease liabilities | 281 | 1,231 | |||
| Derivative liabilities | 6,550 | 3,150 | |||
| Operating lease liabilities, excluding current portion | 19,624 | 21,159 | |||
| Total stockholders' equity | 217,948 | 137,246 | |||
| Total Liabilities and Stockholders' Equity | $ | 273,914 | $ | 188,662 | |
| Common stock outstanding | 77,882,685 | 40,468,141 |
| CONDENSED CONSOLIDATED STATEMENT OF OPERATIONS | Three Months Ended September 30, | Nine Months Ended September 30, | |||||||||||||
| (in thousands, except per share data) | 2025 | 2024 | 2025 | 2024 | |||||||||||
| Operating expenses: | |||||||||||||||
| Research and development | $ | 38,861 | $ | 27,327 | $ | 102,190 | $ | 65,661 | |||||||
| General and administrative | 9,197 | 7,855 | 27,613 | 24,171 | |||||||||||
| Total operating expenses | 48,058 | 35,182 | 129,803 | 89,832 | |||||||||||
| Loss from operations | (48,058 | ) | (35,182 | ) | (129,803 | ) | (89,832 | ) | |||||||
| Other income, net: | |||||||||||||||
| Interest income | 2,586 | 2,328 | 7,852 | 7,544 | |||||||||||
| Interest expense | 336 | (82 | ) | 208 | (265 | ) | |||||||||
| Change in fair value of derivative liabilities | (850 | ) | - | (3,400 | ) | - | |||||||||
| Other income, net | 210 | 211 | 605 | 453 | |||||||||||
| Total other income, net | 2,282 | 2,457 | 5,265 | 7,732 | |||||||||||
| Net loss | $ | (45,776 | ) | $ | (32,725 | ) | $ | (124,538 | ) | $ | (82,100 | ) | |||
| Net loss per share, basic and diluted | $ | (0.48 | ) | $ | (0.79 | ) | $ | (1.46 | ) | $ | (2.04 | ) | |||
| Weighted average shares of common stock outstanding, basic and diluted | 94,417,746 | 41,443,317 | 85,069,288 | 40,182,303 | |||||||||||
Photos accompanying this announcement are available at
This press release was published by a CLEAR® Verified individual.

Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.

















Comments
No comment