
India Declares Three Cough Syrups Toxic After Child Deaths
India has declared three cough syrups as toxic following the deaths of at least 17 children in the past month.
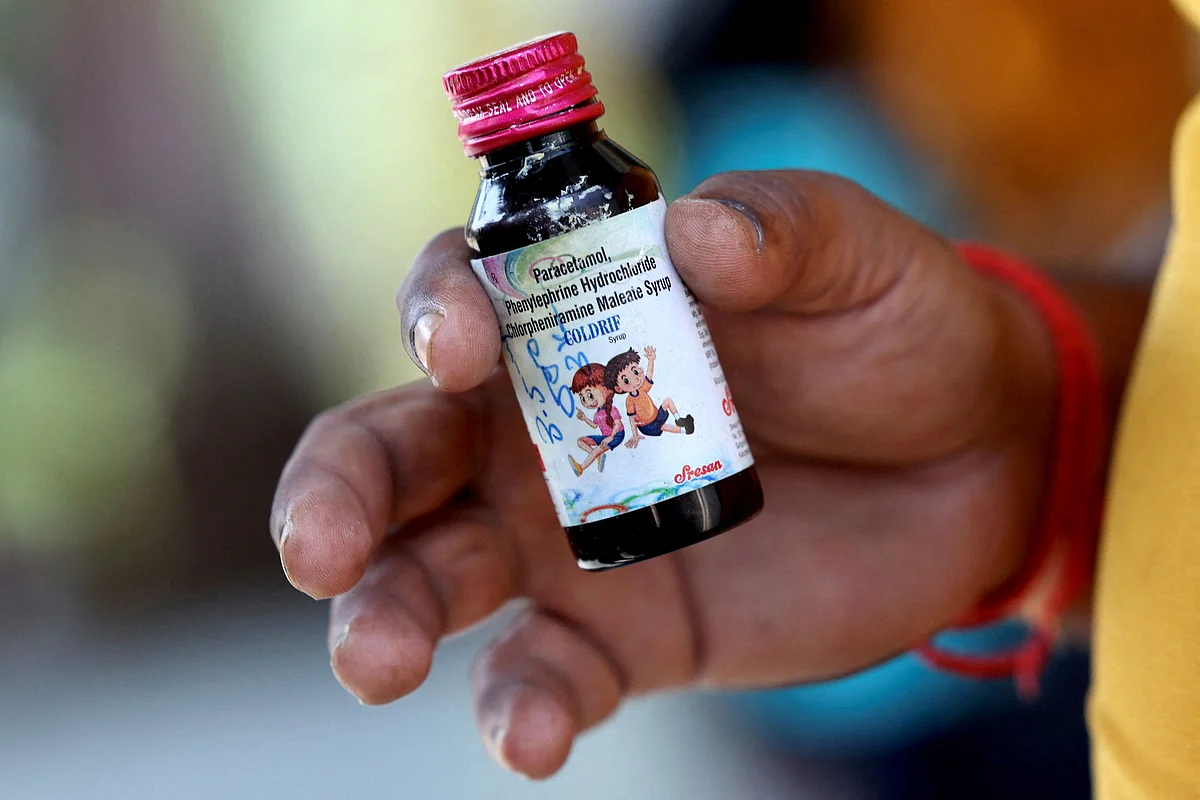
While all the deaths have been linked to Coldrif , regulators in the world's third-largest medicine-producing country have asked consumers to also avoid Respifresh TR and ReLife.
Recommended For YouNone of the syrups have been exported. However, the World Health Organization warned of potential risks through unregulated channels.
Stay up to date with the latest news. Follow KT on WhatsApp Channels.
Here are details of the three tainted cough syrups:
Coldrif
- Linked to all the deaths of the children who came from the central state of Madhya Pradesh. They died of kidney failure, mostly in a government hospital in another state.
Made by Sresan Pharmaceutical Manufacturer, based in the southern state of Tamil Nadu. Contaminated with 48.6 per cent of toxin diethylene glycol (DEG); the permissible limit by India and the WHO is 0.1 per cent.
Made in May 2025 and was set to expire in April 2027. The syrup has been banned and the owner of the company arrested . The owner and the company have not answered repeated calls from Reuters.
Respifresh TR
- Not linked to any deaths.
Made by Rednex Pharmaceuticals, based in the western state of Gujarat. Contaminated with 1.342% DEG.
Made in January 2025 and was set to expire in December 2026. Syrup recalled and the company ordered to stop production of all medical products. Company officials did not respond to calls from Reuters.
ReLife
- Not linked to any deaths.
Made by Shape Pharma, based in Gujarat, in January 2025 and was set to expire in December 2026. Contaminated with 0.616% DEG.
Syrup recalled and the company ordered to stop production of all medical products. Company officials did not respond to calls from Reuters.

Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.
Most popular stories
Market Research

- Tappalpha's Flagship ETF, TSPY, Surpasses $100 Million In AUM
- Nigel Farage To Headline At UK's Flagship Web3 Conference Zebu Live 2025
- PU Prime Launches Halloween Giveaway: Iphones, Watches & Cash Await
- Cregis And Sumsub Host Web3 Compliance And Trust Summit In Singapore
- Luminadata Unveils GAAP & SOX-Trained AI Agents Achieving 99.8% Reconciliation Accuracy
- BTCC Exchange Announces Triple Global Workforce Expansion At TOKEN2049 Singapore To Power Web3 Evolution



















Comments
No comment