
Global Mechanical Thrombectomy Devices Market Worth $ 2.27 Billion By 2030 - Exclusive Report By Insightace Analytics
Global Mechanical Thrombectomy Devices Market info
Global Mechanical Thrombectomy Devices Market seg
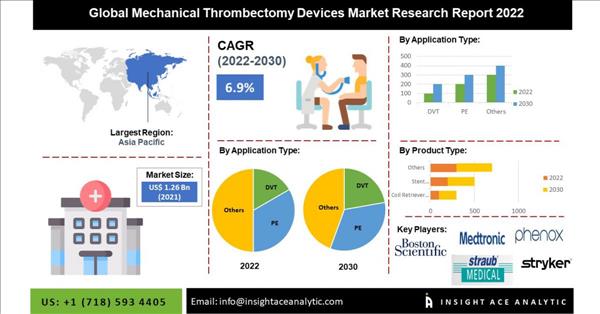
Global mechanical thrombectomy devices market was valued at US$ 1.26 Billion in 2021. It is expected to reach US$ 2.27 Billion by 2030
Major market players operating in the Mechanical Thrombectomy Devices market include Boston Scientific Corporation, Straub Medical AG, AngioDynamics, Acandis GmbH, NIPRO, Medtronic, MicroVention, Inc” — Insightace AnalyticNEW JERSEY, NJ, USA, September 9, 2022 /EINPresswire.com / -- Insightace Analytics Pvt. Ltd. announces the release of a market assessment report on the 'Global Mechanical Thrombectomy Devices Market - by Product Type (Coil Retriever Devices, Stent Retriever Devices, Aspiration Devices, Manual Thrombectomy Devices and Others (Ultrasound Based Devices)), Application Type (Stroke, Deep Vein Thrombosis (DVT), Pulmonary Embolism (PE), Arteriovenous fistula (AVF), Peripheral Arterial Disease (PAD), Haemodialysis Treatments, Percutaneous coronary intervention (PCI) and Others), End-User (Hospitals, Ambulatory Surgical Centers, Specialty Clinics and Academic & Research Institutes), Trends, Industry Competition Analysis, Revenue and Volume Forecast To 2030.'
Request for Sample Pages:
According to the latest research by Insightace Analytics, the global mechanical thrombectomy devices market was valued at US$ 1.26 Billion in 2021. It is expected to reach US$ 2.27 Billion by 2030, with a CAGR of 6.9 % during a forecast period of 2022-2030.
An interventional radiologist will use specialized equipment to perform a mechanical thrombectomy, a minimally invasive procedure, to remove a clot from a patient's artery. A mechanical thrombectomy device removes clots from a vein during an interventional procedure. It is typically only employed as a last option since continuous obstruction of blood flow to organs might result in a condition called necrosis. These tools will minimize the damage to the vessel wall during surgery. According to the size and distance across and depending on the damaged location, thrombectomy devices are also used to treat pneumonic embolism, deep vein thrombosis, neurovascular thrombosis disorders, and peripheral blood vessels. The most frequently used mechanical thrombectomy techniques are balloon embolectomy, aspiration embolectomy, and surgical embolectomy.
The usage of thrombectomy in surgical facilities and hospitals is projected to be driven by the use of clot retrievers and stents to treat strokes and an increase in the number of vascular disorders. The manufacturers' increasing investment in R&D activities to provide top-notch thrombectomy devices will also fuel the expansion of the global mechanical thrombectomy device market. The market is also being driven by the growing elderly population and the rising demand for minimally invasive (MI) operations and bettering healthcare systems worldwide. Additionally, the top manufacturers are investing more money in R&D projects to produce mechanically advanced thrombectomy devices because of the growing rivalry, which is good news for the industry. In addition, favourable clinical reimbursements and experts' increasing preference for using these devices for image-guided medical operations are anticipated to support market growth. However, the lack of skilled professionals and the challenging nature of using these devices limit the market growth. The high cost of thrombectomy equipment is impeding market expansion. Additionally, the lack of knowledge regarding peripheral vascular diseases in developing nations hinders market growth.
North America is anticipated to majorly contribute to the mechanical thrombectomy devices market over the forecast years because several technologically advanced and innovative mechanical thrombectomy devices are nearby. The growth of the North American mechanical thrombectomy devices market is being driven by factors such as the growing use of technologically advanced thrombectomy products among specialists, the rising number of clinical preliminaries and clinical repayments available for thrombectomy systems in the U.S. In addition, the Asia Pacific mechanical thrombectomy devices market is expected to grow significantly during the forecast period due to the increase in cardiovascular illnesses, rising healthcare costs, and gadget technical improvements. The market segment's expansion in this area will be fueled by the rising use of mechanical thrombectomy procedures and medical reimbursement services provided by various central governments.
Major market players operating in the Mechanical Thrombectomy Devices market include Boston Scientific Corporation, Straub Medical AG, AngioDynamics, Acandis GmbH, NIPRO, Medtronic, MicroVention, Inc., Medrad Inc. (Bayer HealthCare LLC), Johnson & Johnson, Rapid Medical Inc., and Anaconda Biomed SL, Imperative Care Inc., Stryker Corporation, Phenox GmbH, Penumbra, Inc., Balt Extrusion, Vascular Medcure, Inc., and Others.
Recent collaborations and agreements in the market:
•In February 2022, Zoom POD Aspiration Tubing, Imperative Care, Inc.'s most recent advancement in improving stroke care, was introduced. The company's Zoom Stroke Solution's newest innovation enables speedy confirmation of clot retrieval success during mechanical thrombectomy for ischemic stroke.
•In October 2021, To treat acute large vessel occlusive (LVO) stroke, phenox developed the pRESET 6-50 mechanical thrombectomy device.
Curious about this latest version of the report? Obtain Report Details @
Market Segments
Global Mechanical Thrombectomy Devices Market, by Product Type, 2022-2030 Value (US$ Mn) and Volume (No. of Units)
•Coil Retriever Devices
•Stent Retriever Devices
•Aspiration Devices
•Manual Thrombectomy Devices
•Others (Ultrasound Based Devices)
Global Mechanical Thrombectomy Devices Market, by Application Type, 2022-2030 Value (US$ Mn) and Volume (No. of Units)
•Stroke
•Deep Vein Thrombosis (DVT)
•Pulmonary Embolism (PE)
•Arteriovenous fistula (AVF)
•Peripheral Arterial Disease (PAD)
•Haemodialysis Treatments
•Percutaneous coronary intervention (PCI)
•Others
Global Mechanical Thrombectomy Devices Market, by End-User, 2022-2030 Value (US$ Mn) and Volume (No. of Units)
•Hospitals
•Ambulatory Surgical Centers
•Specialty Clinics
•Academic & Research Institutes
Global Mechanical Thrombectomy Devices Market, by Region, 2022-2030 Value (US$ Mn) and Volume (No. of Units)
•North America
•Europe
•Asia Pacific
•Latin America
•Middle East & Africa
North America Mechanical Thrombectomy Devices Market, by Country, 2022-2030 Value (US$ Mn) and Volume (No. of Units)
•U.S.
•Canada
Europe Mechanical Thrombectomy Devices Market, by Country, 2022-2030 Value (US$ Mn) and Volume (No. of Units)
•Germany
•France
•Italy
•Spain
•Russia
•Rest of Europe
Asia Pacific Mechanical Thrombectomy Devices Market, by Country, 2022-2030 Value (US$ Mn) and Volume (No. of Units)
•India
•China
•Japan
•South Korea
•Australia & New Zealand
Latin America Mechanical Thrombectomy Devices Market, by Country, 2022-2030 Value (US$ Mn) and Volume (No. of Units)
•Brazil
•Mexico
•Rest of Latin America
Middle East & Africa Mechanical Thrombectomy Devices Market, by Country, 2022-2030 Value (US$ Mn) and Volume (No. of Units)
•GCC Countries
•South Africa
•Rest of Middle East & Africa
Why should buy this report:
To receive a comprehensive analysis of the prospects for the global Mechanical Thrombectomy Devices market
To receive an industry overview and future trends in the Mechanical Thrombectomy Devices market
To analyze the Mechanical Thrombectomy Devices market drivers and challenges
To get information on the Mechanical Thrombectomy Devices market value (US$Mn) and Volume (No. of Units) forecast to 2030
TO get information on investments, mergers & acquisitions in the Mechanical Thrombectomy Devices market industry
For More Information @
Priyanka Tilekar
Insightace Analytic Pvt. Ltd.
+1 551-226-6109
email us here

Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.

















Comments
No comment