
403
Sorry!!
Error! We're sorry, but the page you were looking for doesn't exist.
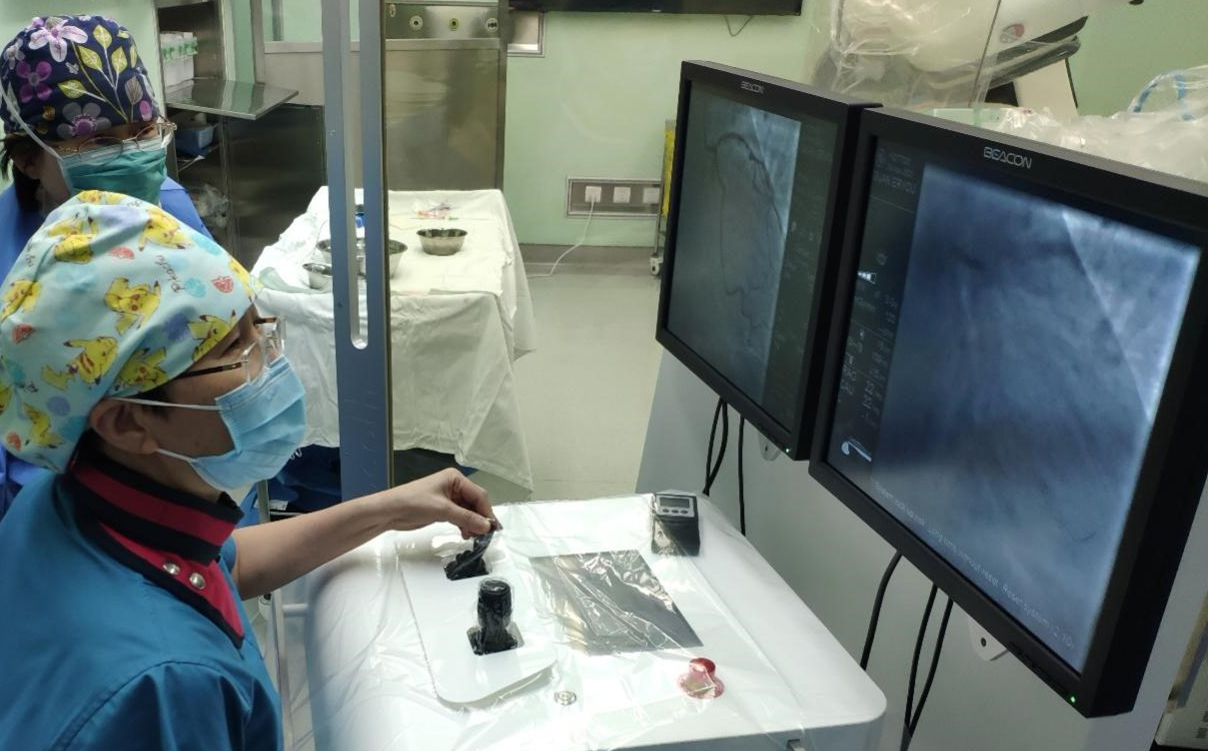
Robocath successfully completes first robotic coronary angioplasty with R One in China
(MENAFN- Andrew Lloyd & Associates) Rouen, France, November 30, 2021 – Robocath, a company that designs, develops and commercializes innovative robotic platforms for the treatment of vascular diseases, today announces the successful completion of the first coronary angioplasty in China assisted by its R-One™ robotic platform. The procedure took place at the 301 Hospital in Beijing on November 24; performed by Dr. Yundai Chen, a renowned interventional cardiologist and author of several scientific publications.
This robotic assisted procedure is the first intervention within a clinical study carried out by CathBot; a joint venture between Robocath and MedBot® (the robot-focused subsidiary of MicroPort Scientific Corporation). This unprecedented clinical study will include over 100 patients, across four centers. The goal is to obtain marketing authorization for the R-One robotic platform in China, via the NMPA (National Medical Products Administration).
Dr. Yundai Chen, interventional cardiologist at the 301 Hospital, said: “Robocath’s robotic platform enables a coronary angioplasty to be carried out safely, whilst being totally protected from radiation, and under more comfortable conditions. It is intuitive and the system is incredibly precise; it’s possible to position stents to the exact millimeter. I’m flattered to have been the first person to use this technology in China and to help push forward the progress of robotics in our country.”
Dr. He Chao, president of MedBot, said: “This first clinical trial of a robotic PCI with R-One is an important milestone in the surgical robotics field for our company. There is no similar product available in our country. With the clinical development and subsequent launch of R-One, it will fill the field gap in robotic PCI in China and will benefit both doctors and patients.”
Philippe Bencteux, president and founder of Robocath and president of CathBot, outlined: “We have reached a key step in our product development, in accordance with our development plan, in a highly strategic geographical area. China currently carries out more angioplasties than any other country. I’d like to thank the CathBot teams for all their work. Together, with our remote treatment technology, our robot will eventually become a key element in access to patient care within the country.”
Lucien Goffart, Robocath’s CEO, added: “In the interventional cardiology field, the Chinese market currently has the highest growth rate in the world. More than a million procedures per year are performed in the country and the number of catheterization labs has doubled since 2010 – now reaching 2,000. This procedure comes only one year after the joint venture setup; it marks a key step in its development. This puts us in a strong position to be awarded marketing authorization from the NMPA in the near future.”
ABOUT MEDBOT
Founded in 2014, MedBot® develops intelligent surgical robotic systems and solutions. MedBot is committed to meeting the most cutting-edge development needs in minimally invasive surgery and innovatively providing integrated intelligent surgical solutions that can save patients’ lives or improve their quality of life. Following years of research and development, innovation and industrial accumulation, MedBot has grown to become a medical robotics company that masters the underlying technology in the entire chain. MedBot’s three flagship products in the three major segments, namely the Toumai™ laparoscopic surgical robot, Skywalker™ joint replacement surgical robot and DFVision™ three-dimensional electronic laparoscope, have entered the special approval procedure (Green Path) for innovative medical devices at the National Medical Products Administration (NMPA); it has become the only surgical robotics company with three ‘Green Path’ grants in the People’s Republic of China (PRC). Its current business covers five areas, including endoscopy, orthopaedics, vascular intervention, natural orifice and percutaneous puncture.
ABOUT MICROPORT
MicroPort® was founded in 1998 in ZJ Hi-Tech Park in Shanghai, China, when a group of dedicated individuals joined together in the common belief that advancements in medical technology could transform patients’ lives in China and around the globe. Over the last two decades, MicroPort has taken important steps towards fulfilling its mission of providing access to the best means of prolonging and improving lives.
Today, MicroPort is focused on covering ten major areas, including cardiovascular intervention & structural heart diseases, electrophysiology & cardiac rhythm management, orthopedics & soft tissue repair, endovascular & peripheral vascular diseases, neurovascular intervention & neurosciences, life sciences (endocrine management), surgical devices & medical robotics, urology & gynecology & respiratory & gastroenterology, aesthetics & rehabilitation, and in vitro diagnostics & medical imaging.
Thanks to over 300 MicroPort devices currently approved for use in nearly 10,000 hospitals worldwide, one of our devices is used every six seconds. With a vast global footprint of R&D and manufacturing sites (Shanghai; Memphis, TN in the United States; Clamart in France; Saluggia in Italy; Santo Domingo in the Dominican Republic), a strong focus on technology innovation with over 4,700 patent applications and a global workforce of over 7,000 employees, MicroPort is committed to its vision of building a people centric consortium of companies focused on emerging medical technologies.
ABOUT ROBOCATH
Founded in 2009 by Philippe Bencteux, MD, Robocath designs, develops and commercializes robotic solutions to treat cardiovascular diseases. As an active player in the evolving medical robotics industry, these innovative solutions aim to make medical procedures safer thanks to reliable technologies, while complementing manual interventions.
R-One™ is the first solution developed by Robocath. It uses a bionic and unique technology that optimizes the safety of robotic-assisted coronary angioplasty. This medical procedure consists of revascularizing the cardiac muscle by inserting one or more implants (stents) into the arteries that supply it with blood. Every 30 seconds, somewhere in the world, this type of procedure is performed. R-One is designed to operate with precision and perform specific movements, creating better interventional conditions. Thanks to its open architecture, R-One is compatible with market-leading devices and cath labs.
In a prospective, randomized, controlled pre-clinical trial, R-One demonstrated safety and efficacy as it achieved 100% technical procedure success and no MACE (major adverse cardiovascular events).
R-One received the CE marking in February 2019 and started its clinical application in September 2019. Currently R-One is available in Europe and Africa.
Robocath aims to become the world leader in vascular robotics and develop the remote treatment of vascular emergencies, guaranteeing the best care pathway for all. Based in Rouen, France, Robocath has more than 60 employees.
This robotic assisted procedure is the first intervention within a clinical study carried out by CathBot; a joint venture between Robocath and MedBot® (the robot-focused subsidiary of MicroPort Scientific Corporation). This unprecedented clinical study will include over 100 patients, across four centers. The goal is to obtain marketing authorization for the R-One robotic platform in China, via the NMPA (National Medical Products Administration).
Dr. Yundai Chen, interventional cardiologist at the 301 Hospital, said: “Robocath’s robotic platform enables a coronary angioplasty to be carried out safely, whilst being totally protected from radiation, and under more comfortable conditions. It is intuitive and the system is incredibly precise; it’s possible to position stents to the exact millimeter. I’m flattered to have been the first person to use this technology in China and to help push forward the progress of robotics in our country.”
Dr. He Chao, president of MedBot, said: “This first clinical trial of a robotic PCI with R-One is an important milestone in the surgical robotics field for our company. There is no similar product available in our country. With the clinical development and subsequent launch of R-One, it will fill the field gap in robotic PCI in China and will benefit both doctors and patients.”
Philippe Bencteux, president and founder of Robocath and president of CathBot, outlined: “We have reached a key step in our product development, in accordance with our development plan, in a highly strategic geographical area. China currently carries out more angioplasties than any other country. I’d like to thank the CathBot teams for all their work. Together, with our remote treatment technology, our robot will eventually become a key element in access to patient care within the country.”
Lucien Goffart, Robocath’s CEO, added: “In the interventional cardiology field, the Chinese market currently has the highest growth rate in the world. More than a million procedures per year are performed in the country and the number of catheterization labs has doubled since 2010 – now reaching 2,000. This procedure comes only one year after the joint venture setup; it marks a key step in its development. This puts us in a strong position to be awarded marketing authorization from the NMPA in the near future.”
ABOUT MEDBOT
Founded in 2014, MedBot® develops intelligent surgical robotic systems and solutions. MedBot is committed to meeting the most cutting-edge development needs in minimally invasive surgery and innovatively providing integrated intelligent surgical solutions that can save patients’ lives or improve their quality of life. Following years of research and development, innovation and industrial accumulation, MedBot has grown to become a medical robotics company that masters the underlying technology in the entire chain. MedBot’s three flagship products in the three major segments, namely the Toumai™ laparoscopic surgical robot, Skywalker™ joint replacement surgical robot and DFVision™ three-dimensional electronic laparoscope, have entered the special approval procedure (Green Path) for innovative medical devices at the National Medical Products Administration (NMPA); it has become the only surgical robotics company with three ‘Green Path’ grants in the People’s Republic of China (PRC). Its current business covers five areas, including endoscopy, orthopaedics, vascular intervention, natural orifice and percutaneous puncture.
ABOUT MICROPORT
MicroPort® was founded in 1998 in ZJ Hi-Tech Park in Shanghai, China, when a group of dedicated individuals joined together in the common belief that advancements in medical technology could transform patients’ lives in China and around the globe. Over the last two decades, MicroPort has taken important steps towards fulfilling its mission of providing access to the best means of prolonging and improving lives.
Today, MicroPort is focused on covering ten major areas, including cardiovascular intervention & structural heart diseases, electrophysiology & cardiac rhythm management, orthopedics & soft tissue repair, endovascular & peripheral vascular diseases, neurovascular intervention & neurosciences, life sciences (endocrine management), surgical devices & medical robotics, urology & gynecology & respiratory & gastroenterology, aesthetics & rehabilitation, and in vitro diagnostics & medical imaging.
Thanks to over 300 MicroPort devices currently approved for use in nearly 10,000 hospitals worldwide, one of our devices is used every six seconds. With a vast global footprint of R&D and manufacturing sites (Shanghai; Memphis, TN in the United States; Clamart in France; Saluggia in Italy; Santo Domingo in the Dominican Republic), a strong focus on technology innovation with over 4,700 patent applications and a global workforce of over 7,000 employees, MicroPort is committed to its vision of building a people centric consortium of companies focused on emerging medical technologies.
ABOUT ROBOCATH
Founded in 2009 by Philippe Bencteux, MD, Robocath designs, develops and commercializes robotic solutions to treat cardiovascular diseases. As an active player in the evolving medical robotics industry, these innovative solutions aim to make medical procedures safer thanks to reliable technologies, while complementing manual interventions.
R-One™ is the first solution developed by Robocath. It uses a bionic and unique technology that optimizes the safety of robotic-assisted coronary angioplasty. This medical procedure consists of revascularizing the cardiac muscle by inserting one or more implants (stents) into the arteries that supply it with blood. Every 30 seconds, somewhere in the world, this type of procedure is performed. R-One is designed to operate with precision and perform specific movements, creating better interventional conditions. Thanks to its open architecture, R-One is compatible with market-leading devices and cath labs.
In a prospective, randomized, controlled pre-clinical trial, R-One demonstrated safety and efficacy as it achieved 100% technical procedure success and no MACE (major adverse cardiovascular events).
R-One received the CE marking in February 2019 and started its clinical application in September 2019. Currently R-One is available in Europe and Africa.
Robocath aims to become the world leader in vascular robotics and develop the remote treatment of vascular emergencies, guaranteeing the best care pathway for all. Based in Rouen, France, Robocath has more than 60 employees.
Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.

















Comments
No comment