
Dräger Expands Neonatal Care Portfolio With Bilipredics Software Solution
-
Enhanced Discharge Planning: Supports clinicians to help reduce unnecessary treatments, tests, and extended hospital stays through predictive insights into bilirubin progression.
Data-Driven Decision Making: Utilizes an extensive database of over 50,000 individual bilirubin measurements of close to 10,000 newborns with a total of about 100,000 individual patient characteristics. Integrated Jaundice Management: Helps provide a streamlined pathway for screening, monitoring, and treating neonatal jaundice.
Advanced predictive capabilities
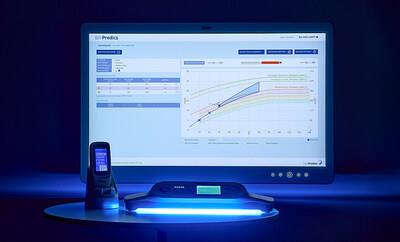
Neonatal jaundice affects approximately 50% of all newborns and up to 80% of premature babies.1 BiliPredics offers a proactive approach to jaundice management by forecasting bilirubin progression over 30, 48, or 60 hours. The web-based application presents this data in an accessible dashboard, helping to enable clinicians to implement preventive measures and reduce the risk of complications.
Smooth integration with clinical workflows
BiliPredics requires only a few clinical parameters to generate comprehensive insights. It aligns with established guidelines, including the 2022 AAP Hyperbilirubinemia Guideline, and can integrate with electronic medical records (EMR) to display patient data within the dashboard. The system presents bilirubin trends through comprehensible curves, helping to allow for real-time assessment and intervention.
Strategic partnership to advance neonatal care
"Through our partnership with NeoPredics, we are expanding our offering with an innovative digital solution that strengthens our position as a leader in jaundice management," said Harald Kneuer, director of neonatal care at Dräger. "By combining Dräger's market expertise with NeoPredics' advanced predictive analytics, we are providing healthcare professionals with powerful tools to improve patient outcomes while managing costs."
NeoPredics CEO, Thorsten Waloschek, echoed this sentiment: "Our collaboration with Dräger is a significant milestone in advancing neonatal care. Together, we are bridging innovative technology and clinical expertise to address critical challenges in jaundice management worldwide."
Comprehensive jaundice management solutions
Dräger's neonatal care portfolio includes solutions for both screening and treatment of neonatal jaundice. The Dräger Jaundice Meter JM-105 offers non-invasive, rapid bilirubin measurement, while the BiliLuxTM Phototherapy Light provides effective LED-based phototherapy for jaundice treatment. This approach helps ensure adherence to the latest guidelines, reinforcing the concept of "phototherapy as a drug" with precise irradiance dosing.
About Dräger
Dräger is a leading international medical and safety technology company. Our products protect, support and save lives. Founded in 1889, Dräger generated global sales of around EUR 3.4 billion in 2023. The Lübeck-based company is represented in more than 190 countries and employs more than 16,000 people worldwide. For more information, visit
NeoPredics
NeoPredics is a leader in predictive analytics and clinical decision support, with a focus on maternal and neonatal health. Committed to improving outcomes for women and children, NeoPredics develops state-of-the-art solutions like the FDA-registered and CE-certified Bilipredics jaundice management solution, addressing critical challenges in care with innovation and precision. For more information, visit
Note: The solution mentioned in this press release will not initially be available in all countries. For more information on the availability of products in your country, please visit the respective country website or contact the local Dräger sales organization.
1Jardine LA, Woodgate P: Neonatal jaundice: phototherapy, ClinicalEvidence 2015;05:319, BMJ Publishing Group
Contact
Communications: Melanie Kamann, Tel. +49 451 882-3202, [email protected]
Press Contact North America: Laura Edwards, Tel. +1 215 565-5868, [email protected]
Investor Relations: Thomas Fischler, Tel. +49 451 882-2685, [email protected]
SOURCE Draeger

Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.














Comments
No comment