
Abu Dhabi Becomes Global First To Administer Itvisma Gene Therapy
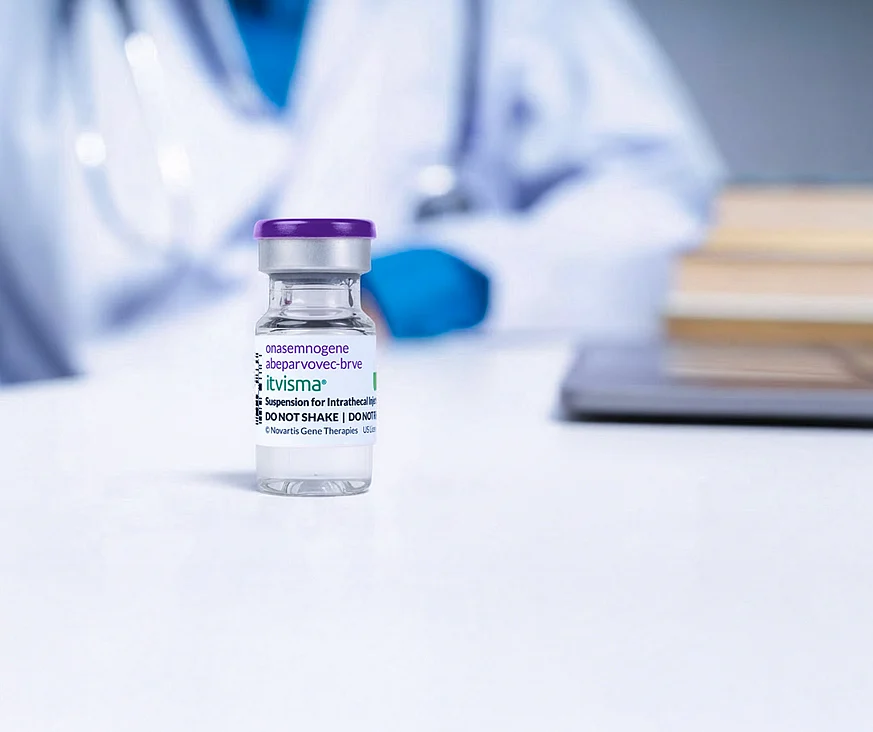
Abu Dhabi marks a landmark achievement in becoming the first in the world to deliver ITVISMA (onasemnogene abeparvovec) for the treatment of spinal muscular atrophy (SMA).
Sheikh Khalifa Medical City (SKMC), part of SEHA, a subsidiary of PureHealth, successfully administered this groundbreaking gene therapy developed by Novartis, under the supervision of the Department of Health – Abu Dhabi (DoH), the regulator of the healthcare sector in the emirate.
Recommended For YouStay up to date with the latest news. Follow KT on WhatsApp Channels.
This groundbreaking therapy received accelerated approval in the UAE on 25th November 2025, positioning the UAE among the first countries globally – after the USA – to endorse this pioneering treatment. This underscores Abu Dhabi's commitment to bringing cutting-edge innovations to patients across the region.
ITVISMA is a one-time gene therapy specifically designed to target the underlying genetic cause of SMA in patients aged 2 years and older with a confirmed SMN1 gene mutation.
Designed for simplicity and long-term impact, ITVISMA replaces the missing SMN1 gene to improve motor function, reducing the need for continuous treatments required by other therapies.
Dr Noura Khamis Al Ghaithi, Under-Secretary of DoH, said,“By administering ITVISMA, we are proud to be among the first to provide this innovative treatment, further reinforcing our role as a leader and accelerator in advanced and innovative healthcare."
Bader Al Qubaisi, Chief Executive Officer at SKMC, said,“Delivering the world's first ITVISMA treatment at SKMC is a testament to Abu Dhabi's integrated healthcare ecosystem under the leadership of the Department of Health – Abu Dhabi.”

Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.

















Comments
No comment