
403
Sorry!!
Error! We're sorry, but the page you were looking for doesn't exist.
Decentralized Clinical Trials Market Size to Reach USD 19.38 billion in 2032
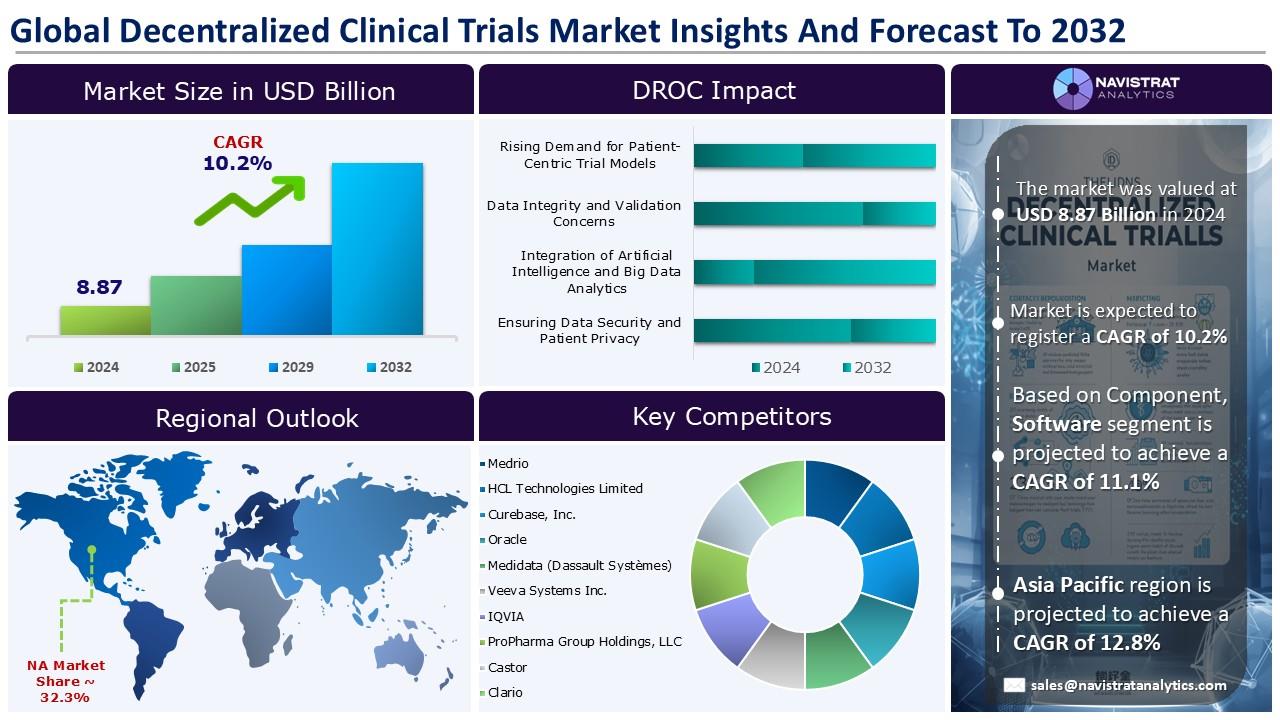
(MENAFN- Navistrat Analytics) December 09, 2025- Growing demand for patient-centric clinical models is a major factor accelerating revenue in the decentralized clinical trials (DCT) market. DCTs are reshaping the research landscape by offering a faster, more economical, and reliable way to conduct studies. Because they allow remote participation, these trials expand accessibility and improve patient experience—enabling sponsors to engage more diverse populations. As global healthcare systems push for equity, efficiency, and better patient engagement, DCTs are emerging as a pivotal model that prioritizes participant needs without compromising scientific rigor.
In June 2025, Medable Inc.—a leading clinical development technology provider—launched its Partner Program to equip CROs and strategic partners with generative AI-powered, self-service eCOA development tools for digital trials. Medable’s SaaS platform has already supported more than 300 decentralized or digitally enhanced studies across 70 countries, connecting with over one million patients and participants globally.
Although DCTs offer substantial advantages, they also introduce operational challenges for sponsors, CROs, and clinical sites. Notably, decentralized models tend to show higher rates of protocol deviations compared with traditional site-based trials, underscoring the need for robust governance and digital infrastructure.
Segments market overview and growth Insights
Based on component, the decentralized clinical trials market is segmented into hardware & devices, software platforms, and support services. Hardware & devices segment contributed the largest revenue share in 2024. Advancements in digital health technologies (DHTs) have broadened the scope of remotely collected clinical trial data, reducing or eliminating the need for patients to travel to centralized research sites. Many wearable devices used in decentralized clinical trials—including fitness trackers, smartwatches, and specialized medical wearables—continuously capture health information and transmit it to paired smartphones or tablets via Bluetooth. In February 2025, Tasso, Inc., a leader in patient-focused, clinical-grade blood collection solutions, introduced its next-generation dried blood spot (DBS) collection technology. The new solution integrates the company’s Tile-T20 dried whole blood cartridge with the Tasso Mini device, enabling precise, user-friendly DBS sampling for clinical studies and anti-doping programs.
Regional market overview and growth insights
North America held the largest market share in 2024. The market’s growth is primarily fueled by increasing adoption of patient-focused trial models and rapid technological progress in digital health systems. Regulatory bodies worldwide have begun recognizing and supporting a shift toward more digitally enabled clinical research. In March 2023, IQVIA Consumer Health and ObvioHealth launched a fully decentralized clinical trial with Jovie USA to support the introduction of the first FDA-approved goat milk-based infant formula in the U.S. The study is the first of its kind in infant nutrition, allowing parents and infants to complete the trial entirely from home with no required in-person visits.
Competitive Landscape and Key Competitors
The Decentralized Clinical Trials market is characterized by a fragmented structure, with many competitors holding a significant share of the market. List of major players included in the Decentralized Clinical Trials market report are:
oMedrio
oHCL Technologies Limited
oCurebase, Inc.
oOracle
oMedidata (Dassault Systèmes)
oVeeva Systems Inc.
oIQVIA
oProPharma Group Holdings, LLC
oCastor
oClario
oHangzhou Tigermed Technology Co., LTD.
oICON plc
oCMIC HOLDINGS Co., LTD.
oThermo Fisher Scientific Inc.
oParexel International Corporation
Major strategic developments by leading competitors
Walgreens: In August 2024, Walgreens partnered with the Biomedical Advanced Research and Development Authority (BARDA), part of the U.S. Department of Health and Human Services, to advance decentralized clinical trial innovation under the D-COHRe (Decentralized Clinical Operations for Healthcare and Research) program.
Labcorp: In January 2024, Labcorp and Hawthorne Effect announced a strategic collaboration aimed at enhancing decentralized clinical trial offerings for pharmaceutical, biotech, and medical device organizations seeking to accelerate recruitment, improve patient diversity, and reduce burden on clinical sites.
Navistrat Analytics has segmented the decentralized clinical trials market based on component, modality, trial phase, therapeutic area, end-use, and region:
•Component Outlook (Revenue, USD Billion; 2022-2032)
oHardware & Devices
oSoftware Platforms
oSupport Services
•Modality Outlook (Revenue, USD Billion; 2022-2032)
oHybrid
oFully decentralized
•Trial Phase Outlook (Revenue, USD Billion; 2022-2032)
oPhase I
oPhase II
oPhase III
oPhase IV
•Therapeutic Area Outlook (Revenue, USD Billion; 2022-2032)
oOncology
oCardiology
oNeurology
oInfectious diseases
oRare diseases
oOthers
•End-Use Outlook (Revenue, USD Billion; 2022-2032)
oPharmaceutical & Biotech sponsors
oContract Research Organizations (CROs)
oMedical device manufacturers
oAcademic and Research Institutions
oOthers
•Regional Outlook (Revenue, USD Billion; 2022-2032)
oNorth America
a.U.S.
b.Canada
c.Mexico
oEurope
a.Germany
b.France
c.U.K.
d.Italy
e.Spain
f.Benelux
g.Nordic Countries
h.Rest of Europe
oAsia Pacific
a.China
b.India
c.Japan
d.South Korea
e.Oceania
f.ASEAN Countries
g.Rest of APAC
oLatin America
a.Brazil
b.Rest of LATAM
oMiddle East & Africa
a.GCC Countries
b.South Africa
c.Israel
d.Turkey
e.Rest of MEA
In June 2025, Medable Inc.—a leading clinical development technology provider—launched its Partner Program to equip CROs and strategic partners with generative AI-powered, self-service eCOA development tools for digital trials. Medable’s SaaS platform has already supported more than 300 decentralized or digitally enhanced studies across 70 countries, connecting with over one million patients and participants globally.
Although DCTs offer substantial advantages, they also introduce operational challenges for sponsors, CROs, and clinical sites. Notably, decentralized models tend to show higher rates of protocol deviations compared with traditional site-based trials, underscoring the need for robust governance and digital infrastructure.
Segments market overview and growth Insights
Based on component, the decentralized clinical trials market is segmented into hardware & devices, software platforms, and support services. Hardware & devices segment contributed the largest revenue share in 2024. Advancements in digital health technologies (DHTs) have broadened the scope of remotely collected clinical trial data, reducing or eliminating the need for patients to travel to centralized research sites. Many wearable devices used in decentralized clinical trials—including fitness trackers, smartwatches, and specialized medical wearables—continuously capture health information and transmit it to paired smartphones or tablets via Bluetooth. In February 2025, Tasso, Inc., a leader in patient-focused, clinical-grade blood collection solutions, introduced its next-generation dried blood spot (DBS) collection technology. The new solution integrates the company’s Tile-T20 dried whole blood cartridge with the Tasso Mini device, enabling precise, user-friendly DBS sampling for clinical studies and anti-doping programs.
Regional market overview and growth insights
North America held the largest market share in 2024. The market’s growth is primarily fueled by increasing adoption of patient-focused trial models and rapid technological progress in digital health systems. Regulatory bodies worldwide have begun recognizing and supporting a shift toward more digitally enabled clinical research. In March 2023, IQVIA Consumer Health and ObvioHealth launched a fully decentralized clinical trial with Jovie USA to support the introduction of the first FDA-approved goat milk-based infant formula in the U.S. The study is the first of its kind in infant nutrition, allowing parents and infants to complete the trial entirely from home with no required in-person visits.
Competitive Landscape and Key Competitors
The Decentralized Clinical Trials market is characterized by a fragmented structure, with many competitors holding a significant share of the market. List of major players included in the Decentralized Clinical Trials market report are:
oMedrio
oHCL Technologies Limited
oCurebase, Inc.
oOracle
oMedidata (Dassault Systèmes)
oVeeva Systems Inc.
oIQVIA
oProPharma Group Holdings, LLC
oCastor
oClario
oHangzhou Tigermed Technology Co., LTD.
oICON plc
oCMIC HOLDINGS Co., LTD.
oThermo Fisher Scientific Inc.
oParexel International Corporation
Major strategic developments by leading competitors
Walgreens: In August 2024, Walgreens partnered with the Biomedical Advanced Research and Development Authority (BARDA), part of the U.S. Department of Health and Human Services, to advance decentralized clinical trial innovation under the D-COHRe (Decentralized Clinical Operations for Healthcare and Research) program.
Labcorp: In January 2024, Labcorp and Hawthorne Effect announced a strategic collaboration aimed at enhancing decentralized clinical trial offerings for pharmaceutical, biotech, and medical device organizations seeking to accelerate recruitment, improve patient diversity, and reduce burden on clinical sites.
Navistrat Analytics has segmented the decentralized clinical trials market based on component, modality, trial phase, therapeutic area, end-use, and region:
•Component Outlook (Revenue, USD Billion; 2022-2032)
oHardware & Devices
oSoftware Platforms
oSupport Services
•Modality Outlook (Revenue, USD Billion; 2022-2032)
oHybrid
oFully decentralized
•Trial Phase Outlook (Revenue, USD Billion; 2022-2032)
oPhase I
oPhase II
oPhase III
oPhase IV
•Therapeutic Area Outlook (Revenue, USD Billion; 2022-2032)
oOncology
oCardiology
oNeurology
oInfectious diseases
oRare diseases
oOthers
•End-Use Outlook (Revenue, USD Billion; 2022-2032)
oPharmaceutical & Biotech sponsors
oContract Research Organizations (CROs)
oMedical device manufacturers
oAcademic and Research Institutions
oOthers
•Regional Outlook (Revenue, USD Billion; 2022-2032)
oNorth America
a.U.S.
b.Canada
c.Mexico
oEurope
a.Germany
b.France
c.U.K.
d.Italy
e.Spain
f.Benelux
g.Nordic Countries
h.Rest of Europe
oAsia Pacific
a.China
b.India
c.Japan
d.South Korea
e.Oceania
f.ASEAN Countries
g.Rest of APAC
oLatin America
a.Brazil
b.Rest of LATAM
oMiddle East & Africa
a.GCC Countries
b.South Africa
c.Israel
d.Turkey
e.Rest of MEA
Navistrat Analytics
Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.
















Comments
No comment