
FDA Expands Indication For Levita Magnetics' Magnetic Surgical System With MARS® For Minimally Invasive Bariatric And Hiatal Hernia Repair
"This expanded clearance is a major step forward. With the improved grasper and magnetic liver retraction, I can now perform complex bariatric procedures and hiatal hernia repairs with greater visibility and fewer incisions," said Dr. Chad Carlton, a Dallas-based surgeon and long-time MARS user. "It's a smarter, less invasive way to care for patients-especially those with high BMI and challenging anatomy, with less pain."
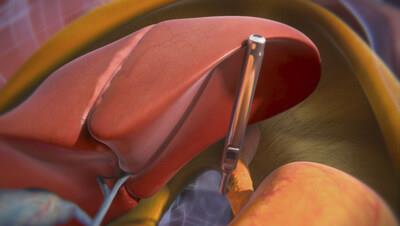
Unlike other robotic systems that often rely on rigid instrumentation and multiple incisions, the MARS platform introduces a new paradigm: using proprietary magnetic technology to reposition organs internally. This innovation allows surgeons to reduce the number of incisions, minimize tissue trauma, and enhance patient recovery, all while maintaining surgical precision and control.
"This milestone reinforces our commitment to expanding access to better surgery," said Dr. Alberto Rodriguez-Navarro, founder and CEO of Levita Magnetics. "We're building a future where less invasive means more effective."
Commercial Momentum and Market Opportunity
Hiatal hernia repair is a common procedure, frequently performed alongside bariatric surgery in patients with obesity-related conditions. Levita's newly cleared indication allows the MARS system to better support this high-need patient population, offering improved access and visualization through magnetic retraction and an updated toolset.
With the addition of this indication and the new 12.5mm grasper, Levita is expanding its addressable market and strengthening its commercial offerings for surgical teams managing complex abdominal cases.
Pioneering the Future of Magnetically Assisted Surgeries
Levita's continued innovation has opened new frontiers in surgical care. From enabling groundbreaking bariatric procedures to supporting surgeries designed to improve quality of life, the company's MARS platform is redefining what's possible in the operating room. With a growing body of clinical use cases and national media attention highlighting patient outcomes-from dramatic weight loss to minimally invasive cancer-related procedures, Levita is helping surgeons deliver transformative results across a wide spectrum of care.
Levita also continues to expand its clinical presence nationwide and internationally and expects to install systems at multiple top-tier institutions in the short term.
For media inquiries or to request an interview with Dr. Carlton or Dr. Rodriguez-Navarro, please contact:
Tim Gray
Communications Strategist
917-202-9515
[email protected]
About Levita Magnetics
Levita Magnetics is a Silicon Valley-based medical device company pioneering --minimally invasive platforms that use magnetics to improve surgical precision while reducing invasiveness. Its flagship MARS system provides surgeons with enhanced control and visualization while minimizing the trauma of traditional surgery. The MARS platform is FDA-approved for gallbladder, bariatric, prostate, colorectal, and now hiatal hernia procedures.
SOURCE Levita Magnetics

Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.

















Comments
No comment