
Stryker Receives FDA Clearance For Optablate® BVN Basivertebral Nerve Ablation System
Key features of the system include:
-
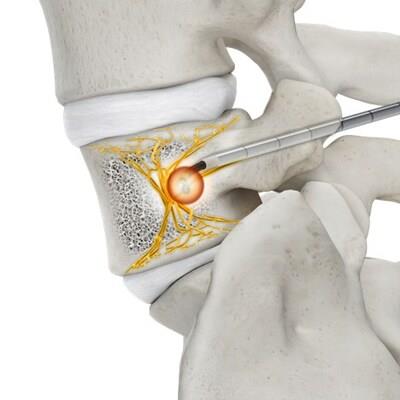
Achieves at least a 1 cm lesion in 7 minutes 2
Steerable, dynamic curved introducer for targeted performance 2
Microinfusion technology, which keeps the zone hydrated, reducing impedance errors and preventing charring2
10-gauge access tools
"We have a long history in radiofrequency ablation, and we're relentlessly committed to delivering groundbreaking approaches to protect and promote quality of life," said Kristen Berg, vice president and general manager of Stryker's Interventional Spine business. "Our legacy providing solutions to reduce pain now continues with OptaBlate BVN, addressing the underserved population suffering from chronic vertebrogenic lumbar pain."
Stryker's Interventional Spine business will introduce OptaBlate BVN as part of its pain portfolio at the American Society of Pain & Neuroscience (ASPN) Annual Meeting, July 17-20, in Miami, FL. (Booth #154).
For questions, please click here .
About Stryker
Stryker is a global leader in medical technologies and, together with its customers, we are driven to make healthcare better. We offer innovative products and services in MedSurg, Neurotechnology and Orthopaedics that help improve patient and healthcare outcomes. Alongside our customers around the world, we impact more than 150 million patients annually. More information is available at .
Media contact
Beth Sizemore
Senior Director, Communications
[email protected]
*Chronic low back pain of at least six months duration that has not responded to at least six months of conservative care
†The evidence shows patients treated with BVNA had sustained benefits in pain and function for up to 5 years
References
Fischgrund JS, Rhyne A, Macadaeg K, Moore G, Kamrava E, Yeung C, Truumees E, Schaufele M, Yuan P, DePalma M, Anderson DG, Buxton D, Reynolds J, Sikorsky M. Long-term outcomes following intraosseous basivertebral nerve ablation for the treatment of chronic low back pain: 5-year treatment arm results from a prospective randomized double-blind sham-controlled multi-center study. Eur Spine J. 2020 Aug;29(8):1925-1934. doi: 10.1007/s00586-020-06448-x. Epub 2020 May 25. PMID: 32451777. Stryker data on file.SOURCE Stryker

Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.

















Comments
No comment