
403
Sorry!!
Error! We're sorry, but the page you were looking for doesn't exist.
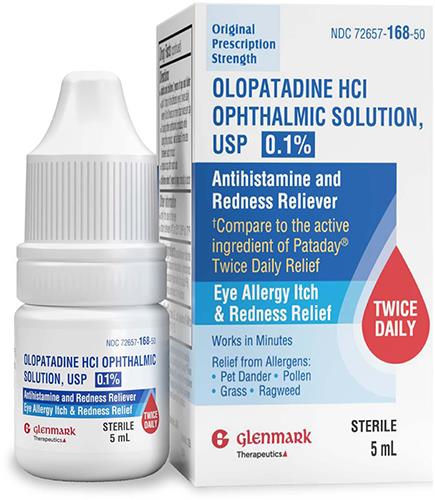
Glenmark Therapeutics Inc., USA launches Olopatadine Hydrochloride Ophthalmic Solution USP, 0.1% (OTC)
(MENAFN- Adfactors PR) Bengaluru, August 19, 2024: Glenmark Therapeutics Inc., USA (Glenmark) is pleased to announce it has launched1 Olopatadine Hydrochloride Ophthalmic Solution USP, 0.1% (OTC); compare to the active ingredient in Pataday®2 Twice Daily Relief.
According to Nielsen® syndicated data for the latest 52 weeks’ period ending July 13, 2024, the Pataday® Twice Daily Relief (OTC) market3 achieved annual sales of approximately $26.4 million*.
Commenting on the launch, Fabio Moreno, Head – OTC Sales & Marketing, Glenmark Pharmaceuticals Inc. said, “We are excited to announce the launch of Olopatadine Ophthalmic Solution USP, 0.1%, addressing the growing demand for a new supplier in this category. This addition highlights our commitment to meeting market needs and providing high-quality over- the-counter solutions for our customers.”
According to Nielsen® syndicated data for the latest 52 weeks’ period ending July 13, 2024, the Pataday® Twice Daily Relief (OTC) market3 achieved annual sales of approximately $26.4 million*.
Commenting on the launch, Fabio Moreno, Head – OTC Sales & Marketing, Glenmark Pharmaceuticals Inc. said, “We are excited to announce the launch of Olopatadine Ophthalmic Solution USP, 0.1%, addressing the growing demand for a new supplier in this category. This addition highlights our commitment to meeting market needs and providing high-quality over- the-counter solutions for our customers.”
Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.















Comments
No comment