
FDA Flags Breakdowns At Indian Pharma Factory Exporting To The US
US inspectors have uncovered new and dangerous breakdowns in drugmaking at an Indian factory owned by Sun Pharma that produces generic medications for American consumers.
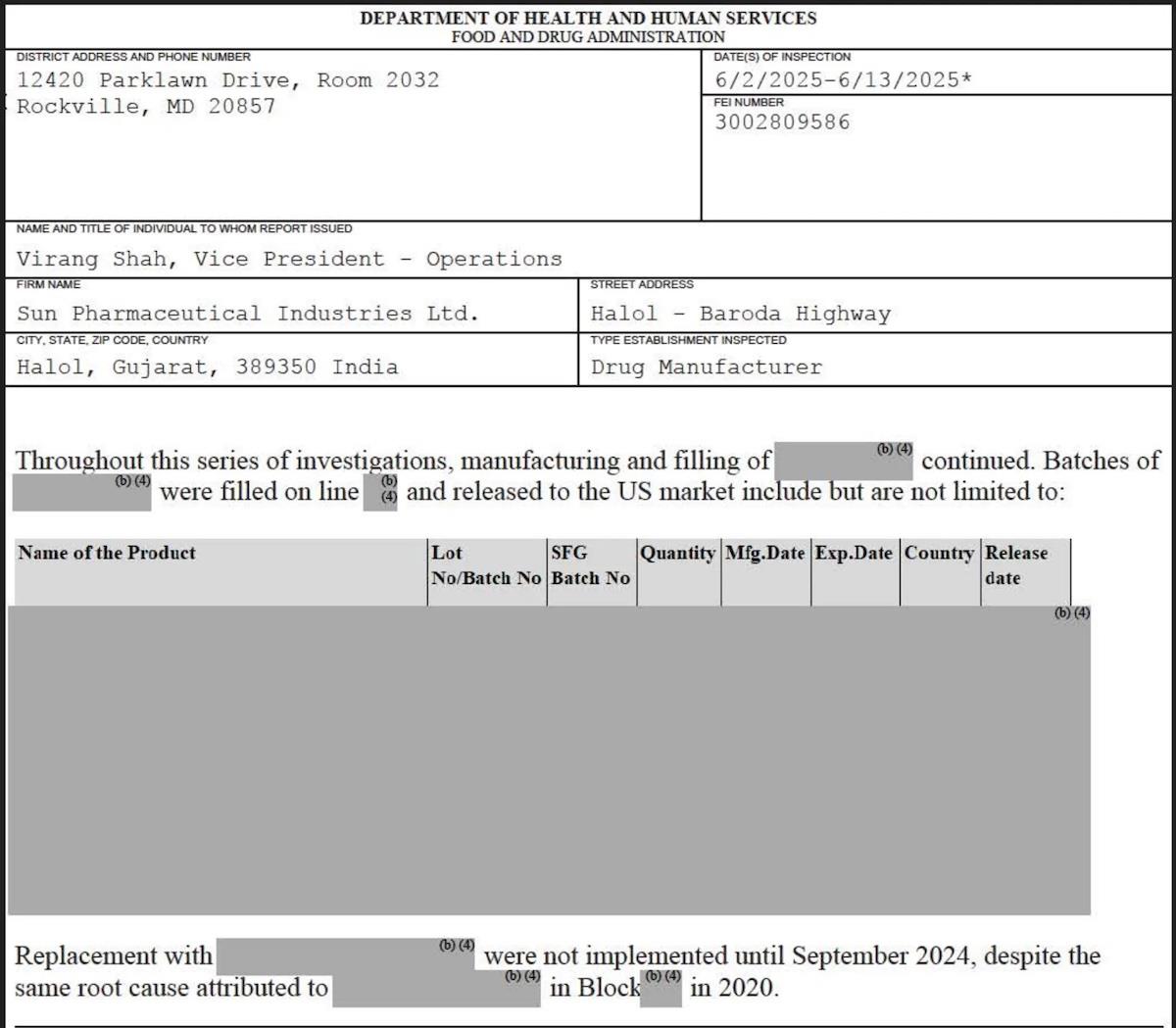
The latest problems come 2 1/2 years after the Food and Drug Administration gave the facility a special pass to continue sending certain drugs made there to the United States, even after the factory was officially banned from the US market.
The factory failed to investigate the source of bacteria found in test vials or deal with damaged equipment that had caused drugs to be contaminated with metal particles, according to the June inspection report , which ProPublica obtained through a Freedom of Information Act request.
Workers improperly handled vials and stoppers meant for sterile medications and, in some cases, failed to disinfect manufacturing areas and equipment, according to the report. One FDA inspector saw a worker put on a sterile gown and then brush up against a waste bin and use their hands to push down the overflowing trash. Investigators also saw liquid dripping through ceiling cracks and the growth of what appeared to be fungus and mold in a storage area for samples used for testing.
The FDA in late 2022 had banned the factory in the city of Halol from shipping drugs to the United States because of similar manufacturing failures.

Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.





















Comments
No comment