
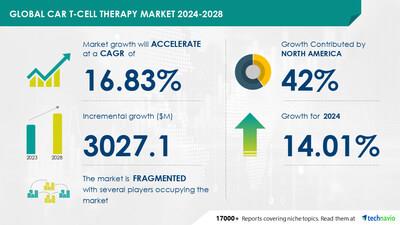
Car T-Cell Therapy Market Size Is Set To Grow By USD 3.02 Billion From 2024-2028, Growing Awareness Regarding Car T-Cell Therapy To Boost The Market Growth, Technavio
| Forecast period |
2024-2028 |
| Base Year |
2023 |
| Historic Data |
2018 - 2022 |
| Segment Covered |
End-user (Hospitals and Cancer treatment centers), Type (CD19, CD22, BCMA, and Others), and Geography (North America, Europe, Asia, and Rest of World (ROW)) |
| Region Covered |
North America, Europe, Asia, and Rest of World (ROW) |
| Key companies profiled |
ACROBIOSYSTEMS INC., Allogene Therapeutics Inc., Autolus Therapeutics plc, Bristol Myers Squibb Co., Celyad Oncology SA, Eli Lilly and Co., Fate Therapeutics Inc., Fortress Biotech Inc., Gilead Sciences Inc., GlaxoSmithKline Plc, Johnson and Johnson Services Inc., Les Laboratoires Servier, Merck KGaA, Miltenyi Biotec B.V. And Co. KG, Noile Immune Biotech Inc., Novartis AG, Pfizer Inc., Sangamo Therapeutics Inc, Sorrento Therapeutics Inc., and TCR2 Therapeutics Inc. |
Key Market Trends Fueling Growth
The global CAR T-cell therapy market is experiencing significant growth due to an increasing number of product approvals and clinical trials. For instance, Yescarta, the first CAR T-cell therapy to receive FDA approval for relapsed or refractory large B-cell lymphoma, was granted approval in February 2022. Similarly, ciltacabtagene autoleucel (Carvykti) for multiple refractory myeloma received FDA approval in the same month. The number of clinical trials for CAR-T cell therapies has surged, with 415 trials registered on Clinicaltrials as of November 23, 2022. These trials cover various stages of development and focus on different types of cancer and patient groups. Supportive government policies, rising research and development expenditure, and the potential to treat various types of cancer are driving the growth of the global CAR T-cell therapy market.
The Car T-cell therapy market is experiencing significant financial backing with companies like Juno Therapeutics and Tessa Therapeutics leading the charge in clinical trials for blood cancer treatments. Juno's T-cell therapy, Tisagenlecleucel, has shown promising results in treating Non-Hodgkin lymphoma and leukemia. Tessa Therapeutics' TT-02 is also making strides in solid tumors, including ovarian cancer. Adaptimmune, Celgene Corporation, and others are focusing on scalability and cost-effectiveness while maintaining product consistency. CAR T-cell therapies are revolutionizing cancer treatment, with precision medicine and tailoring treatments to individual patients becoming increasingly important. Hematologic malignancies, such as leukemia and lymphoma, are the primary indications. Despite the high cost and systemic toxicity, investments continue to pour in due to the potential for a cure. Reimbursement policies, public awareness, patient population, and healthcare infrastructure are key factors influencing market growth. Innovation in cell-based therapies, including genetic modification using CRISPR/Cas9, is driving progress in cancer treatments. The prevalence of cancer and incidence of specific diseases, such as leukemia and lymphoma, continue to fuel demand. Quality control measures are essential to ensure safety and efficacy, making patient-centric approaches crucial for success. With the growing patient population and disposable income, the market for Car T-cell therapies is poised for continued growth.
Discover 360° analysis of this market. For complete information, schedule your consultation-
Book Here!
Market
Challenges
-
The global CAR T-cell therapy market faces a significant challenge due to the high cost of this innovative treatment. The cost is influenced by the complex manufacturing process, personalized nature, and specialized healthcare infrastructure requirements. CAR T-cell therapies involve collecting a patient's T-cells through leukapheresis, modifying them in the lab, expanding their quantity, and reinfusing them back. This process necessitates specialized facilities, equipment, and expertise, contributing to the overall cost. Moreover, each patient's therapy is personalized, adding complexity and cost. Extensive clinical trials and research further increase the cost burden. CAR T-cell acquisition costs range from USD373,000 to USD475,000 per infusion, not including additional procedures or facility fees. The inpatient setting for therapy administration adds an additional cost of USD79,466 to USD85,267. Consequently, the high costs associated with CAR T-cell therapies pose a significant challenge to the market's growth during the forecast period.
The Car T-cell therapy market is experiencing significant growth due to the potential of these treatments for blood cancer, specifically lymphoma and leukemia. Companies like Juno Therapeutics and Tessa Therapeutics are leading the charge with their CAR T-cell therapies, TT-02 for solid tumors, and Tessa's TCR-based therapy for ovarian cancer. However, challenges remain, including securing financial backing for clinical trials, ensuring scalability, cost-effectiveness, and product consistency. The market is also facing hurdles in treating solid tumors and addressing systemic toxicity. Collaborations with companies like Celgene Corporation and innovation through precision medicine are key to overcoming these challenges. Reimbursement policies, public awareness, investments, and patient population size are also crucial factors. Genetic modification through CRISPR/Cas9 holds promise for improving CAR T-cell therapies. Despite these challenges, the high prevalence of cancer and the potential for a cure make this an exciting area for innovation and patient-centric approaches.
For more insights on driver and challenges
-
Download a Sample Report
Segment Overview
This car t-cell therapy market report extensively covers market segmentation by
End-user-
1.1 Hospitals
1.2 Cancer treatment centers
-
2.1 CD19
2.2 CD22
2.3 BCMA
2.4 Others
-
3.1 North America
3.2 Europe
3.3 Asia
3.4 Rest of World (ROW)
1.1 Hospitals-
The hospital segment dominates the global CAR T-cell therapy market due to hospitals being the primary administrators of these treatments. Hospitals provide essential infrastructure, medical staff, and resources for CAR T-cell therapy. Oncologists, hematologists, and nurses manage the treatment process, ensuring patient safety and monitoring response. Hospitals identify eligible patients, assessing medical history, performing diagnostics, and evaluating suitability. Pre-treatment evaluations, including imaging and lab tests, establish baselines and monitor progress. Hospitals closely monitor patients during therapy for adverse reactions and provide post-treatment follow-up care. The growing number of cancer treatments in hospitals is expected to fuel market growth.
For more information on market segmentation with geographical analysis including forecast (2024-2028) and historic data (2018 - 2022)
- Download a Sample Report
Research Analysis
Car T-cell therapy is a revolutionary cancer treatment that utilizes a patient's own immune system to fight cancer cells. This innovative approach involves engineering T-cells to recognize and attack specific cancer antigens, making it a promising solution for various types of cancers, particularly leukemia and lymphoma. The global market for Car T-cell therapy is expected to grow significantly due to the increasing incidence of cancer, prevalence of blood cancer, and advancements in healthcare infrastructure. Reimbursement policies and public awareness are key factors influencing the market's growth. The patient population for cancer treatments is vast, and the precision medicine approach of Car T-cell therapies offered by companies like Celgene Corporation and Juno Therapeutics is tailoring treatments to individual patients, further boosting its adoption. The future of Car T-cell therapy holds great promise in transforming the landscape of cancer treatment.
Market Research Overview
Car T-cell therapy is a revolutionary cancer treatment that involves genetically modifying a patient's T-cells to recognize and attack cancer cells. With the increasing incidence of cancer worldwide, the demand for effective and innovative treatments is on the rise. Reimbursement policies and public awareness are key factors influencing the market growth. Significant investments are being made in this field, driven by the potential for high disposable income and a large patient population. Prevalence of cancer, particularly leukemia and lymphoma, is a significant driver for the market. Innovation in the field is ongoing, with the use of CRISPR/Cas9 technology and patient-centric approaches gaining attention. Financial backing from major corporations such as Celgene Corporation and clinical trials for solid tumors, including ovarian cancer, are also contributing to the market's growth. Scalability, cost-effectiveness, and product consistency are crucial challenges for the industry. Tailoring treatments to individual patients through precision medicine and hematologic malignancies are key indications for CAR T-cell therapies. Immunotherapy, genetic modification, and systemic toxicity are important considerations in the development of these therapies. Quality control measures are essential to ensure safety and efficacy. Companies like Juno Therapeutics, Tessa Therapeutics, and Adaptimmune are leading the way in this field, with TT-02 and other CAR T-cell therapies showing promise for the treatment of various cancers.
Table of Contents:
1 Executive Summary
2 Market Landscape
3 Market Sizing
4 Historic Market Size
5 Five Forces Analysis
6 Market Segmentation
-
End-user
-
Hospitals
Cancer Treatment Centers
-
CD19
CD22
BCMA
Others
-
North America
Europe
Asia
Rest Of World (ROW)
7 Customer Landscape
8 Geographic Landscape
9 Drivers, Challenges, and Trends
10 Company Landscape
11 Company Analysis
12 Appendix
About Technavio
Technavio is a leading global technology research and advisory company. Their research and analysis focuses on emerging market trends and provides actionable insights to help businesses identify market opportunities and develop effective strategies to optimize their market positions.
With over 500 specialized analysts, Technavio's report library consists of more than 17,000 reports and counting, covering 800 technologies, spanning across 50 countries. Their client base consists of enterprises of all sizes, including more than 100 Fortune 500 companies. This growing client base relies on Technavio's comprehensive coverage, extensive research, and actionable market insights to identify opportunities in existing and potential markets and assess their competitive positions within changing market scenarios.
Contacts
Technavio Research
Jesse Maida
Media & Marketing Executive
US: +1 844 364 1100
UK: +44 203 893 3200
Email:
[email protected]
Website:
SOURCE Technavio

Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.

















Comments
No comment