(MENAFN- PR Newswire) pro disc® C SK expands availability of Centinel Spine's portfolio of FDA-approved cervical total disc replacement (TDR) devices. Centinel Spine now has three cervical TDR devices available in the U.S. for 1-level indications: pro disc C , pro disc C Vivo, and pro disc C SK. Centinel Spine offers the broadest spectrum of TDR solutions to address individual patient anatomy and meet surgeon preference needs .
WEST CHESTER, Pa., Nov. 10, 2022 /PRNewswire/ -- Centinel Spine®, LLC, ('the Company') a leading global medical device company addressing cervical and lumbar spinal disease through anterior surgical access, today announced the first implantation of its pro disc® C SK Cervical Total Disc Replacement (TDR) product. In July, the Company received U.S. Food and Drug Administration approval for 1-level indications for pro disc C Vivo , pro disc C SK, and pro disc C Nova . The pro disc C SK system is the second of the three new products to be released with the Company recently announcing the 100th completed procedure with its pro disc C Vivo TDR product. Along with the currently available pro disc C implant, Centinel Spine has the broadest offering of cervical TDR solutions in the world to address individual patient anatomy and meet surgeon preference needs.
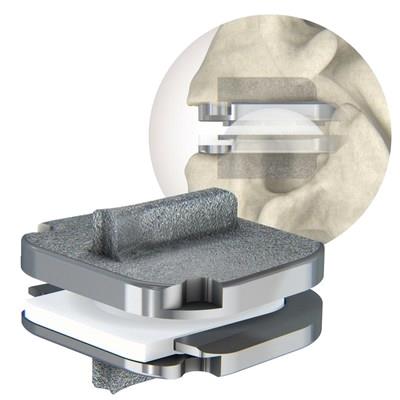
prodisc C SK Cervical Total Disc Replacement Device
'In spine care, each patient's circumstances are different, so it is critical to provide surgeons with the ability to address each patient's unique needs,' said Centinel Spine CEO Steve Murray. 'With the release of both the prodisc C Vivo
and prodisc C SK devices, we have expanded the options available to surgeons to allow for better matching of the disc to the patient. While these two new product releases have been conducted on a limited scale, we have already seen a strong positive response from surgeon partners related to the potential patient benefits.'
The pro disc C SK device features a flat endplate design for optimized implant positioning that allows surgeons to address individual patient anatomy—and a low-profile central keel that provides immediate fixation and enables a streamlined keel preparation technique. Similar to all pro disc products, the pro disc C SK device incorporates pro disc CORE technology, the basis behind the predictable clinical outcomes of the pro disc platform after 30 years and over 225,000 implantations worldwide.*
According to orthopedic spine surgeon Ehsan Jazini, MD, from the Virginia Spine Institute
in Reston, Virginia, 'I am proud to be the first surgeon to use this innovative technology in the DC metro region. As a pioneer working to make disc replacement surgery ultra-customized for each patient, the pro disc C SK and pro disc C Vivo devices provide me the versatility to match the disc to the patient's anatomy. With this advanced proven technology, we can best gain motion preservation and stability as appropriate for each cervical surgery.'
Orthopedic spine surgeon Jason Tinley, MD, founder of the DFW
Center for Spinal Disorders
in Dallas-Fort Worth, Texas, agrees, noting, 'With pro disc C SK now FDA-approved along with pro disc C Vivo and the original
pro disc C , I have the intra-operative modularity to change implant characteristics based on patient morphology. While an MRI or X-ray may appear to favor the need for a flat endplate and keels versus a superior dome shape with spikes, once your carpentry is performed, I've found that it's not uncommon that the alternative option may actually offer better stability and endplate conformity upon trialing.'
* Data on file
About Centinel Spine, LLC Centinel Spine®, LLC is a leading global medical device company
addressing cervical and lumbar spinal disease through anterior surgical access. The company offers a continuum of trusted, brand-name, motion-preserving and fusion solutions backed by over 30 years of clinical success—providing the most robust and clinically-proven technology platforms in the world for total disc replacement (pro disc ®) and Integrated Interbody™ fusion ( STALIF® ).
Centinel Spine continues to advance its pioneering culture and corporate mission to become a catalyst of change in the spine industry and alter the way spine surgery is perceived. Centinel Spine remains the only company with comprehensive motion-preserving and fusion solutions for both cervical and lumbar anterior column reconstruction.
For more information, please visit the company's website at
or contact:
Centinel Spine Varun GandhiChief Financial Officer 900 Airport Road, Suite 3BWest Chester, PA 19380Phone: 484-887-8871Email:
Media Kyle EvansICR WestwickePhone: 646-677-1295Email:
SOURCE Centinel Spine, LLC




















Comments
No comment