
Spinal Resources Inc. Announces Publication In Spine Deformity Of Impact Of A Novel Patient-Specific, Patient-Matched Bezier Parametric Curve Rod Platform On Proximal Junction Biomechanics In An In Silico Thoracolumbar Fusion Model
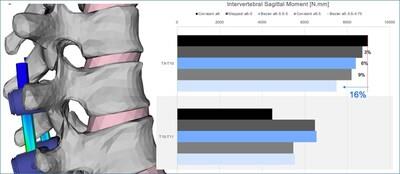
Dr. Theologis continues, "Specifically, in this finite element analysis, it was found that Bezier rods provided smoother load transitions and better offloading of proximal segments compared to constant diameter rods. The highest moment sustained by the segment adjacent to the instrumentation was observed with the constant 6mm rods, while the Bezier 5.5-5-4.75mm rod showed the lowest moment, indicating reduced stress of 16% on the upper adjacent vertebrae (Figure 1). Similarly, the Bezier rods were more effective in offloading pedicle screws up to 45% with respect to the stiffer rod construct. (Figure 2)."
Dr. Saeed S. Sadrameli, M.D., Board Certified in Neurosurgery, specializing in endoscopic and patient-specific minimally invasive/robotic spine surgery, Advent Health points out, "The underlying design principle applies equally to long, complex deformity constructs and shorter segmental fusions, where junctional stress and implant fatigue remain a concern. The Bezier technology marks a shift toward biomechanically optimized and personalized instrumentation, delivering stiffness and compliance precisely tailored to each region of the spine."
Dr. Sadrameli continues, "Tailored to each patient's anatomy and surgical plan, the Bezier rod shows strong potential to mitigate common mechanical complications in spinal fusion, such as Proximal Junctional Kyphosis (PJK), by optimizing load-sharing between the rod and the spine at critical spinal regions."
According to Bernie Bedor, SRI President and CEO, "These are extremely promising and exciting findings that lend strong support for and validate SRI's Bezier Parametric Curve Transition Rods' potential in reducing the risk of PJK/PJF following adult and pediatric spinal deformity operations."
About Spinal Resources Inc.
Spinal Resources Inc.® is a Ft. Lauderdale, Florida based spinal medical device company that supports cost-effective patient care with innovative mechanical and bio-mechanical products to alleviate pain, shorten recovery time, restore health, and extend quality of life.
SOURCE Spinal Resources Inc.

Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.
















Comments
No comment