
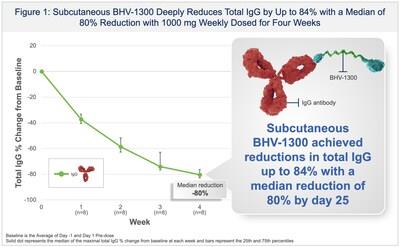
Biohaven Reports Positive Degrader Data Achieving &Gt 80% Sustained Reductions In Total Igg With Potential First-In-Class BHV-1300
Tova Gardin, MD, MPP, Chief Translational Officer at Biohaven, commented, "BHV-1300 has demonstrated remarkable efficacy in deep lowering of total IgG, leveraging the groundbreaking technology of the MoDE platform, to potentially revolutionize treatment of patients with autoimmune disease. Biohaven's unique extracellular degrader technology leverages the body's natural hepatic clearance mechanism to remove targeted antibodies contributing to disease and promises to usher in a new era of tunable, selective and self-administered immune therapy."
BHV-1300 was safe and well-tolerated in subcutaneous doses up to 2000 mg with no clinically significant increases in ALT, AST, or bilirubin, no clinically significant reductions in albumin, and no clinically significant increases in cholesterol over the four-week dosing period compared to placebo. There were no clinically significant reductions in IgG3, IgA, IgE, or IgM compared to baseline. Most AEs were mild and self-resolving, there were no discontinuations due to AEs related to study drug, and there were no serious or severe AEs. The Phase 1 study is ongoing with plans to continue to escalate multiple doses to explore the full range of targeted IgG lowering possible with this technology to customize an ideal treatment approach for different disease indications.
Biohaven's MoDE technology used in developing BHV-1300 was licensed from Yale University stemming from groundbreaking chemistry work in the Spiegel Lab. Yale Professor David Spiegel, MD, PhD, inventor of the MoDE technology and the first to patent the use of targeted extracellular protein degraders that utilize the asialoglycoprotein receptor (ASGPR), commented, "This remarkable demonstration in humans of rapid, deep and sustained reductions of targeted IgG removal with BHV-1300 is a breakthrough and a testament to the scientific advancements that can be accomplished by innovative academic and industry collaborations. BHV-1300 has catapulted the field of extracellular degraders forward and promises to shift the paradigm for the treatment of individuals living with immune-mediated diseases. It is truly an honor to be able to collaborate with the team at Biohaven on this exciting journey."
BHV-1300 is differentiated from monoclonal antibodies targeting FcRn inhibition, offering a novel and selective approach to treat autoimmune causes of disease, while enabling patients to maintain immune protection against infection through preservation of IgG3 (Figure 2). IgG degradation with BHV-1300 is deep and tunable, capable of achieving remarkable depth of IgG lowering, and with refinement in degradation depth feasible through titration of dose level and frequency. It is designed for self-administration via an easy-to-use and patient-friendly autoinjector through an ongoing partnership with Ypsomed AG.
Dr. Gardin added, "This data released today supports advancing BHV-1300 as a potential first-in-class, small molecule approach to treating Graves' disease, a common autoimmune disease that is currently treated with surgery, ablation or anti-thyroid drugs. Our innovative approach unifies cutting-edge science with renewed understanding of disease pathology, to advance a potential first and best-in-class therapeutic for the treatment of Graves' disease. Based on the PK/PD and safety profiles exhibited in the ongoing Phase 1 study, we are thrilled to advance BHV-1300 forward as we aim to disrupt the current treatment paradigm in Graves' disease and potentially revolutionize the treatment of this disease which impacts millions of patients across the world."
Graves' disease is an autoimmune condition that impacts 3 million individuals in the US and 80 million people worldwide. In Graves' disease (Figure 3), IgG1 autoantibodies mimic TSH, binding the TSH receptor in the thyroid and stimulating excess production of thyroid hormones. Graves' disease impacts every organ system, causing symptoms such as cardiac arrhythmias, anxiety, heat intolerance, weight loss, changes in appetite and bowel movements and shortness of breath, in addition to causing related conditions of thyroid eye disease and infiltrative dermopathy. A considerable unmet need exists for improved therapeutic options that target the underlying autoimmune etiology of disease and do not result in permanent hypothyroidism or bear risk of fatal agranulocytosis, hepatotoxicity and teratogenicity. While conventional treatments for Graves' disease, including thyroid removal or ablation and antithyroid drugs, have not changed in 70 years, scientific understanding of immunobiology has advanced considerably, enabling the development of BHV-1300, a precision therapeutic that targets the underlying IgG1 autoantibodies causing the disease.
About BHV-1300
BHV-1300 is a small molecule and potential first-in-class extracellular IgG degrader, rationally designed to leverage the body's natural hepatic clearance mechanisms to selectively target and remove IgG1, IgG2, and IgG4, the underlying cause of the disease. BHV-1300 spares IgG3 to preserve patient immune protection against bacteria, viruses and parasites (Figure 2). The results of the ongoing Phase 1 study confirm that BHV-1300 produces deep reductions in total IgG, is selective, sparing IgG3, is tunable, and is safe and well-tolerated.
About Biohaven
Biohaven is a biopharmaceutical company focused on the discovery, development, and commercialization of life-changing treatments in key therapeutic areas including immunology, neuroscience and oncology. The company is advancing its innovative portfolio of therapeutics, leveraging its proven drug development experience and multiple proprietary drug development platforms. For more information, visit .
Forward-looking Statements
This news release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. The use of certain words, including "continue", "plan", "will", "believe", "may", "expect", "anticipate", "potential first-in-class", "disrupt", "potentially revolutionize", "groundbreaking", "potential first and best-in-class" and similar expressions, is intended to identify forward-looking statements. Investors are cautioned that any forward-looking statements, including statements regarding the future development, timing and potential marketing approval and commercialization of development candidates, are not guarantees of future performance or results and involve substantial risks and uncertainties. Actual results, developments and events may differ materially from those in the forward-looking statements as a result of various factors including: the expected timing, commencement and outcomes of Biohaven's planned and ongoing clinical trials; the timing of planned interactions and filings with the FDA; the timing and outcome of expected regulatory filings; complying with applicable US regulatory requirements; the potential commercialization of Biohaven's product candidates; and the effectiveness and safety of Biohaven's product candidates. Additional important factors to be considered in connection with forward-looking statements are described in Biohaven's filings with the Securities and Exchange Commission, including within the sections titled "Risk Factors" and "Management's Discussion and Analysis of Financial Condition and Results of Operations". The forward-looking statements are made as of the date of this news release, and Biohaven does not undertake any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
MoDE and TRAP are trademarks of Biohaven Therapeutics Ltd.
Investor Contact:
Jennifer Porcelli
Vice President, Investor Relations
[email protected]
+1 (201) 248-0741
Media Contact:
Mike Beyer
Sam Brown Inc.
[email protected]
+1 (312) 961-2502
SOURCE Biohaven Ltd.

Legal Disclaimer:
MENAFN provides the information “as is” without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the provider above.





















Comments
No comment