
403
Sorry!!
Error! We're sorry, but the page you were looking for doesn't exist.
Stent and Heart-Valve Implantation Boost Medical Polyether Ether Ketone Industry
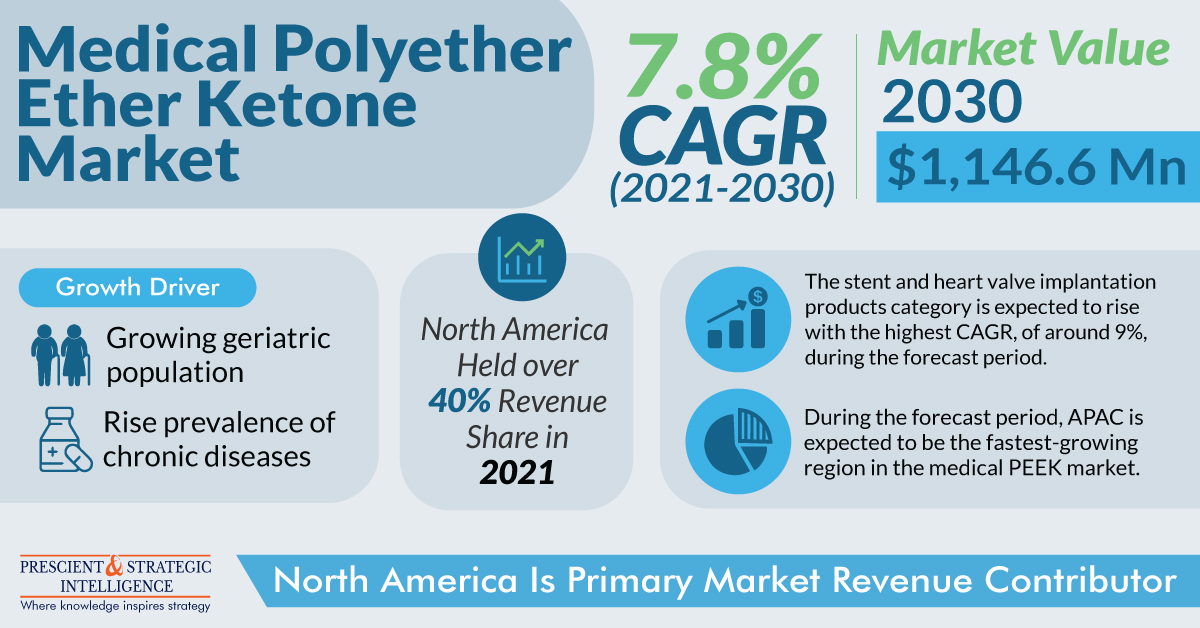
(MENAFN- P&S Intelligence) The medical polyether ether ketone market generated $582.4 million in 2021, and it is expected to rise to $1,146.6 million in 2030, advancing at a rate of 7.8% from 2021 to 2030, ascribed to the massive replacement of the metallic implants made from PEEK, due to increasing government funding to healthcare services and the emergence of private players.
Moreover, high-performance plastic garners have a high preference among materials for trauma fixation, dental implants, cardiovascular devices, and numerous other applications. In addition, the rise in the geriatric population in Asian, North American, and European countries, along with rising sports injuries are expected to boost the market.
The rapid industrialization, and increase in the research activities in the developing countries, more preferably in the APAC region boost the medical polyether ether ketone (PEEK) market, due to an increase in the manufacturing of PEEK-based medical products. In addition, the major players in the market are focusing on a range of strategic plans for business development, such as investments, partnerships, and product launches to advance their position in the market. Thus, it has resulted in high-grade polymers for devices & medical implants.
For instance, CircumFix Solutions garnered an investment by Evonik Industries AG for a sternal closure device in February 2022. Similarly, Maxx Orthopedics Inc. and Invibio Ltd. partnered together for the first total knee arthroplasty by PEEK femoral knee component in July 2021.
Furthermore, the stent and heart valve implantation products are expected to experience a significant rise in the medical polyether ether ketone (PEEK) market, accounting for 9% in the coming years. Moreover, PEEK is massively being used in the manufacturing of a large number of cardiovascular devices and implants such as cannulae, hear-valve implants, and stents. The medical-grade plastic is highly used due to its electric isolation, and its modified mechanical performance in minimally invasive cardiac surgeries.
Moreover, the growing prevalence of heart-related issues, such as heart attacks, strokes, and coronary artery diseases leads to medical polyether ether ketone (PEEK) market proliferation. Around 0.7 million people in the U.S. die due to heart diseases, and more than 0.8 million people experience a heart attack each year. In addition, dental implants massively use PEEK as a titanium substituent, and they are expected to witness a steady increase at the rate of 8.5% in the near future in the market.
Therefore, the semi-crystallin engineering thermoplastics usage for trauma fixation, orthopedic, orthodontic, and cardiovascular applications is projected to expand the medical polyether ketone market. Furthermore, the increasing investments in the healthcare industry driven by technological advancements are predicted to cause a market boom.
North America contributes significant revenue to the medical PEEK market, ascribed to the expansion of healthcare facilities, and innovations in medical devices. Moreover, the surging requirement for stents, heart valves, spine implants, and knee and hip implants led by the growing population in the country, results in an increase in medical-grade plastics usage.
Moreover, the penetration of the medical devices and component manufacturers that utilize PEEK in the country boosts the market. Along with this, the presence of major manufacturing companies in the U.S. drives the region’s market.
Therefore, the increasing use of PEEK as titanium substituent in dental implants and prevalence of the cardiac diseases propel the market.
Moreover, high-performance plastic garners have a high preference among materials for trauma fixation, dental implants, cardiovascular devices, and numerous other applications. In addition, the rise in the geriatric population in Asian, North American, and European countries, along with rising sports injuries are expected to boost the market.
The rapid industrialization, and increase in the research activities in the developing countries, more preferably in the APAC region boost the medical polyether ether ketone (PEEK) market, due to an increase in the manufacturing of PEEK-based medical products. In addition, the major players in the market are focusing on a range of strategic plans for business development, such as investments, partnerships, and product launches to advance their position in the market. Thus, it has resulted in high-grade polymers for devices & medical implants.
For instance, CircumFix Solutions garnered an investment by Evonik Industries AG for a sternal closure device in February 2022. Similarly, Maxx Orthopedics Inc. and Invibio Ltd. partnered together for the first total knee arthroplasty by PEEK femoral knee component in July 2021.
Furthermore, the stent and heart valve implantation products are expected to experience a significant rise in the medical polyether ether ketone (PEEK) market, accounting for 9% in the coming years. Moreover, PEEK is massively being used in the manufacturing of a large number of cardiovascular devices and implants such as cannulae, hear-valve implants, and stents. The medical-grade plastic is highly used due to its electric isolation, and its modified mechanical performance in minimally invasive cardiac surgeries.
Moreover, the growing prevalence of heart-related issues, such as heart attacks, strokes, and coronary artery diseases leads to medical polyether ether ketone (PEEK) market proliferation. Around 0.7 million people in the U.S. die due to heart diseases, and more than 0.8 million people experience a heart attack each year. In addition, dental implants massively use PEEK as a titanium substituent, and they are expected to witness a steady increase at the rate of 8.5% in the near future in the market.
Therefore, the semi-crystallin engineering thermoplastics usage for trauma fixation, orthopedic, orthodontic, and cardiovascular applications is projected to expand the medical polyether ketone market. Furthermore, the increasing investments in the healthcare industry driven by technological advancements are predicted to cause a market boom.
North America contributes significant revenue to the medical PEEK market, ascribed to the expansion of healthcare facilities, and innovations in medical devices. Moreover, the surging requirement for stents, heart valves, spine implants, and knee and hip implants led by the growing population in the country, results in an increase in medical-grade plastics usage.
Moreover, the penetration of the medical devices and component manufacturers that utilize PEEK in the country boosts the market. Along with this, the presence of major manufacturing companies in the U.S. drives the region’s market.
Therefore, the increasing use of PEEK as titanium substituent in dental implants and prevalence of the cardiac diseases propel the market.

Legal Disclaimer:
MENAFN provides the
information “as is” without warranty of any kind. We do not accept
any responsibility or liability for the accuracy, content, images,
videos, licenses, completeness, legality, or reliability of the information
contained in this article. If you have any complaints or copyright
issues related to this article, kindly contact the provider above.
Most popular stories
Market Research
- Thinkmarkets Adds Synthetic Indices To Its Product Offering
- Ethereum Startup Agoralend Opens Fresh Fundraise After Oversubscribed $300,000 Round.
- KOR Closes Series B Funding To Accelerate Global Growth
- Wise Wolves Corporation Launches Unified Brand To Power The Next Era Of Cross-Border Finance
- Lombard And Story Partner To Revolutionize Creator Economy Via Bitcoin-Backed Infrastructure
- FBS AI Assistant Helps Traders Skip Market Noise And Focus On Strategy



















Comments
No comment