
FDA Clearance Issued For Revolutionary Magnetic Compression Surgical Technique Soon To Launch In U.S. Market
MINNEAPOLIS, Oct. 29, 2024 /PRNewswire/ -- GT Metabolic Solutions Inc., the global leader in magnetic compression anastomosis surgery, announced today that the U.S. Food and Drug Administration (FDA) cleared GT Metabolic's MagDITM
System for side-to-side duodeno-ileal (DI) anastomosis.1 The MagDITM System is the first-of-its-kind minimally invasive surgical technique to create anastomosis without foreign materials left behind.2-4
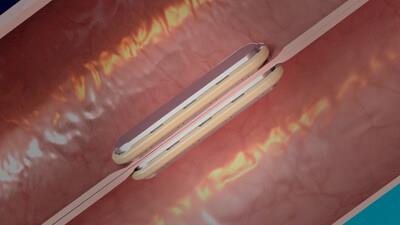
Self-aligning GT Metabolic MagDITM System magnets fused together
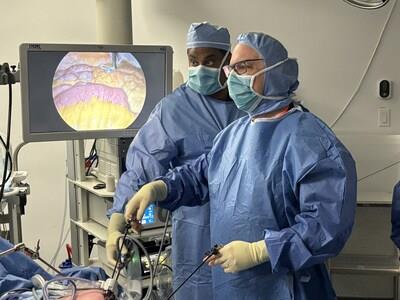
Pictured: U.S. bariatric surgeon Paul Enochs, MD, alongside GT Metabolic MagDITM System inventor Michel Gagner, MD, perform a side-to-side duodeno-ileal (DI) anastomosis
"With this FDA clearance, the MagDITM System is poised to launch a new frontier in healthcare as we continue to deliver on our visionary milestones for efficacy in magnetic surgery," said MedTech entrepreneur Thierry Thaure, CEO and co-founder of GT Metabolic. "After completing over 100 cases in seven clinical trials across eight countries, launching in the U.S. market with this groundbreaking technology is a major progression in our journey to advance minimally invasive surgery in the bariatric space as well as in future areas."
The MagDITM System is composed of the GT Metabolic linear DI magnets, the GT Metabolic delivery system, and the GT Metabolic laparoscopic positioning device (LPD). During the MagDI procedure two linear magnets are delivered orogastrically to the patient. The magnets are positioned laparoscopically and self-align through the small bowel. After several weeks the magnets compress the tissue fusing together forming an anastomosis. The magnets detach and are expressed naturally.
The anastomosis created with magnet compressions happens without the cutting or piercing of intestinal tissue that occurs with the current practice of stapling or suturing. The MagDITM System is designed for more consistent tissue alignment, central necrosis, and circumferential healing while leaving no foreign materials behind to impede the natural tissue healing process.2-4
The MagDITM System was cleared after clinical data submitted to the FDA showed the system performed as intended. In all subjects, the MagDITM
System created patent side-to-side duodeno-ileal anastomosis. There were no reports of anastomotic bleeding, leakage, or obstruction.1
"It's a paradigm shift creating a new standard of care that democratizes anastomosis creation," said inventor Michel Gagner, MD, FRCSC, FACS, and chief medical officer and co-founder of GT Metabolic. "We're providing the surgical community with a novel approach to minimally invasive surgery shown to have zero bleeds and zero leaks. Suturing and stapling bowel tissue for anastomosis creation will become outdated. Magnetic compression anastomosis technology will revolutionize the industry."
Prominent U.S. bariatric surgeon Paul Enochs, MD, FACS, FASMBS, of Bariatric Specialists of the Carolinas was
the first U.S. surgeon to have patients enrolled in clinical trials using the MagDITM System. "Seeing the science and technology that's behind this procedure has definitely opened my eyes to what the future could hold," said Enochs.
GT Metabolic is currently identifying key sites for additional clinical studies. Key opinion leaders in the bariatric surgery specialty interested in participating in the MagDITM System launch can contact the company at GT Metabolic .
About GT Metabolic TM
Solutions Inc.
GT Metabolic Solutions Inc. is leading the world with its development of magnetic compression anastomosis technologies for next-stage minimally invasive procedures. An elegant approach that uses magnets to help achieve anastomosis, the incisionless, sutureless, staple-free technique leaves no foreign material to impede the natural tissue healing process.
Working in tandem with renowned global experts, our team has engineered a magnetic compression solution called delayed anastomosis technology (DAT) that surgeons can use to create consistent anastomosis while helping minimize potential complications, such as leaks and bleeds, in challenging applications. Our solution democratizes the surgical approach to anastomosis. It can be used in procedure staging and is 100% reversible.
Committed to improving patients' lives and healthcare provider outcomes, GT Metabolic Solutions Inc. is disrupting the market by introducing magnetic compression anastomosis technologies to bariatric, metabolic and digestive health providers in the U.S. and abroad.
510(k) No. K242086. Clearance for Magnetic Compression Anastomosis System (MagDI System) issued by the U.S. Food and Drug Administration. 24 October 2024.Gagner, M., Almutlaq, L., Cadiere, G.-B., Torres, A. J., Sanchez-Pernaute, A., Buchwald, J. N., & Abuladze, D. (2023). Side-to-side magnetic duodeno-ileostomy in adults with severe obesity with or without type 2 diabetes: Early outcomes with prior or concurrent sleeve gastrectomy.
Surgery for Obesity and Related Diseases.
Gagner, M., Abuladze, D., Koiava, L.
et al. First-in-Human Side-to-Side Magnetic Compression Duodeno-ileostomy with the Magnet Anastomosis System.
OBES SURG
33, 2282–2292 (2023).
Gagner M, Cadiere GB, Sanchez-Pernaute A, Abuladze D, Krinke T, Buchwald JN, Van Sante N, Van Gossum M, Dziakova J, Koiava L, Odovic M, Poras M, Almutlaq L, Torres AJ. Side-to-side magnet anastomosis system duodeno-ileostomy with sleeve gastrectomy: early multi-center results. Surg Endosc. 2023 Aug;37(8):6452-6463. doi: 10.1007/s00464-023-10134-6. Epub 2023 May 22. PMID: 37217682; PMCID: PMC10202352.
Legal Disclaimer / Forward-Looking Statements
This release is for informational purposes only and is not an offer to sell securities or a solicitation of an offer to buy any securities and may not be related upon in connection with the purchase or sale of any security. This release may contain forward-looking statements regarding projected business performance, operating results, financial condition and other aspects of the company. These statements also include estimates of the technology developments, adoption of the company's products and improvement of patient outcomes. Please be advised that such statements are estimates only and there is no assurance that the results stated or implied by forward-looking statements will actually be realized by the company. Forward-looking statements may be based on management assumptions that prove to be wrong. The company's predictions may not be realized for a variety of reasons, including due to competition, hospital sales cycles, and engineering or technical issues, among others. The company and its business are subject to substantial risks and potential events beyond its control that would cause material differences between predicted results and actual results, including the company incurring operating losses and experiencing unexpected material adverse events.
Learn more about magnetic compression anastomosis and GT MetabolicTM Solutions, Inc.
Contact for US Medical Community and Technology:
Ronald Galovich, VP Sales & Marketing
GT Metabolic Solutions, Inc.
[email protected]
Contact for Investment and International:
Thierry Thaure, Chief Executive Officer, Co-Founder
GT Metabolic Solutions, Inc.
[email protected]
SOURCE GT Metabolic Solutions
WANT YOUR COMPANY'S NEWS FEATURED ON PRNEWSWIRE? 440k+Newsrooms &
Influencers 9k+
Digital Media
Outlets 270k+
Journalists
Opted In GET STARTED

Legal Disclaimer:
MENAFN provides the information “as is” without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the provider above.





















Comments
No comment